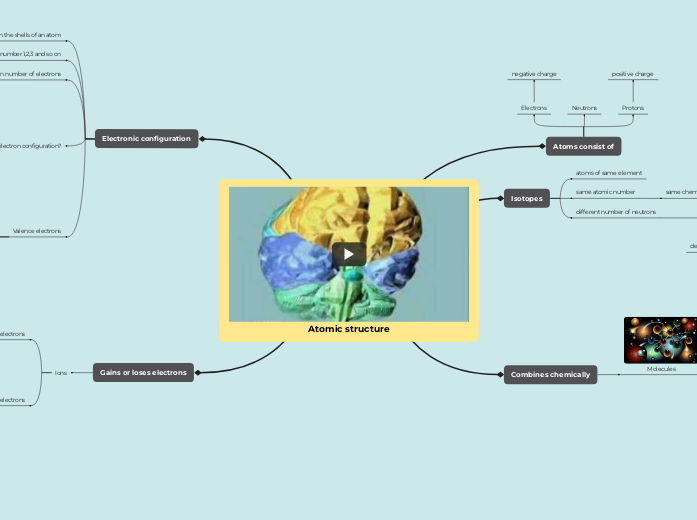
Atomic structure
Gains or loses electrons
Ions
Lose electrons
ions are positively charged
metals
cations
Gain electrons
ions are negatively charged
non-metals
anions
Electronic configuration
Valence electrons
nature of elements determined by the number of valence electrons
4-8 non-metalic
1-2 metallic
aliminium with 3 is an exception
outermost shell=shell furthest from the nucleus
find in outermost occupied shell of an atoms
How to find electron configuration?
electrons start occupying new shells when previous ones are occupied
arrange electrons in shells
find the derived number of electrons
find the proton number of the atom
each shell can occupy a certain number of electrons
shells number 1,2,3 and so on
electrons arranged around the nucleus in the shells of an atom
Combines chemically
Molecules
of elements
atoms of same elements
phosphorus
chlorine
oxygen
hydrogen
nitrogen
of compounds
atoms of different elements
hydrochloric acid
carbon dioxide
water
ammonia
methane
Isotopes
different number of neutrons
different physical properties
boilling point
melting point
density
same atomic number
same chemical properties
same electronic configuration of both isotopes
atoms of same element
Atoms consist of
Protons
positive charge
Neutrons
Electrons
negative charge