Biochemistry
Topic 5 - Cells and Organelles
Cell Structure and Functions
Plant-Only Organelles
Chloroplast
Found in Plants and Algae
Site of photosynthesis - the production of glucose using the sun’s energy
Contains stacks (called grana) of pancake-like structures (called thylakoids)
Inner and outer membrane
Contains pigments - especially chlorophyll
Green oval
Cell Wall
Found in Plant cells (also fungi and bacteria)
Structural support and protection
Rigid outer layer, composed of cellulose
Double Membrane-Bound Organelles
Mitochondrion
Releases energy from glucose
Site of aerobic respiration
Has its own DNA
Folded inner membrane (cristae)
Smooth outer membrane
Double-membrane bound
Single Membrane-Bound Organelles
Lysosome
In both Animal and Plant Cells
The digestive enzymes they possess are used to break down a variety of things, including the cell itself!
Membrane balls containing digestive enzymes
Vesicle/Vacuole
Plant cells tend to have one large vacuole, while animal cells will have a few smaller ones, if any
All cells have vesicles
Vacuoles tend to be larger, especially in plants, and are typically used for storage
Vesicles transport materials out of the cell and store materials inside the cell
Literally just membrane balls
Packaging and Transport Organelles
Golgi Body
Modifies proteins
Processes and packages proteins to be sent out of the cell
Stacks of flattened membrane sacs
Endoplastic Reticulum
In all eukaryotes
Transport of molecules within the cell
Rough - embedded with ribosomes
Smooth - no ribosomes attached
Network of tubes/membranes extending outwards from the nucleus to the membrane
Ribosomes
In all cells
Constructs proteins
Made of rRNA and protein
Freely floating in the cytoplasm or attached to the endoplasmic reticulum
Nucleus
Present in all cells in some form
Contains all instructions to govern cell function
DNA/RNA
Nucleolus
Production of ribosomes
Cluster of protein - RNA in the center of the nucleus
Nuclear Envelopment
Both in Animal & Plant Cells - not present in prokaryotic cells
Protect the genetic material inside the nucleus
Single bilayer surrounding the nucleus
Types of Cells
Eukaryote
Plant
Animal
Prokaryote
Cyanobacteria
Bacteria
Common Cell Structures
Cytoplasm
Genetic Material
Cell Theory
Cells only come from pre-existing cells
The cell is the smallest unit of life
All living things are composed of cells or cell products
Topic 4 - Enzymes
4.2 - Enzyme Function
Feedback Inhibition
Occurs when a product from one of the reaction inhibits a previous step
Can be competitive or non-competitive inhibition
Can take place when there is a series of enzyme catalyzed reactions, where a product from each reaction becomes part of the next reaction
Competitive Inhibition
The inhibitor molecule is similar in shape to the substrate, and can bind directly to the active site - substrate is now blocked from binding
Allosteric Regulation
An allosteric site on an enzyme is any site that is NOT the active site. It can be used as an on-off switch
If a cell wants the ability to turn on and off a particular enzyme. Allosteric regulation is one mechanism it can use.
More complex method of controlling enzyme activity
Factors Affecting Enzyme Activity
Enzyme Co-Factor
Substances other than the enzyme and substrate. Their job is to help activate the enzyme itself
Coenzymes, which are organic molecules. Their job is to transfer energy in the form of electrons
They include vitamins, and an important molecule we’ll see much more of later called NAD+
Metal ions, such as Ca2+, Zn2+ and Cu2+
pH
Certain enzymes are designed to function within a very narrow range of pH conditions
Temperature
Higher temperature can increase rate of reaction up until the point that it denatures the enzyme
Substrate concentration
The more substrate the there is, the higher the rate of reaction - up to a point
Enzyme Catalyzed Reaction
7 : The active site becomes available for another set of substrates
6 : Product is released
5 : Substrates are converted into new product, new bonds are formed
4 : Enzyme lowers EA
3 : Substrates are held in the active site through weak intermolecular forces like H-bonds
2 : Enzyme changes shape accordingly
1 : Substrates binds to the active site
4.1 - Enzymes
Why rely on enzymes?
Enzymes exhibit substrate specificity - so they only encourage reactions the cell wants
Enzymes are not consumed during the reaction, and are therefore reusable
Heat - for instance, will generally speed up reactions by getting particles to move around faster, increasing the chance that reactants will bump into each other
Strategies to Decrease Activation Energy
S - Straining Bonds
Enzyme pulls on intramolecular bonds causing strain, and making it more likely for them to break with lower input of energy
D - Direct Participation
Enzyme, well, participates directly in the reaction in some way
O - Orientation
Enzyme orients the substrate in such a way that it makes it more likely bonding sites come into contact with one another
M - Microenvironment
Enzyme creates a more suitable mini-environment for the reaction to occur
Energy profile
Endergonic Reaction
Typically Anabolic
Results in an “absorption” of energy
More energy in the products than in the reactants
Exergonic Reaction
Typically Catabolic
Results in an overall release of energy
More energy in the reactants than the products
Free energy - a measure of the energy available to do something
Role :is to speed up biological reactions by lowering activation energy (EA)
Activation energy : energy needed to be added before a reaction can proceed
Is a Biological Catalyst
Topic 3 - Macromolecules
3.8 - Nucleic Acids
Shape of DNA
Bases are joined together with hydrogen bonds
Formation of a Polymer
The bond created is called a phosphodiester bond
When two nucleotides join, the nitrogenous base is not involved. The phosphate group from one nucleotide binds to a hydroxyl group on the sugar of the second nucleotide
General Structure of Nucleotide (Nucleotide - Monomer which make up a Nucleic Acid)
3 Main Components
Phosphate Group
Pentose Sugar (5 carbons)
Ribose in RNA
Deoxyribose in DNA
Nitrogenous Base
Uracil (U)
Thymine (T)
Cytosine (C)
Guanine (G)
Adenine (A)
ATP (adenosine triphosphate) - stores potential energy in phosphate bonds, releases than energy when converted to ADP
RNA (ribonucleic acid) - translates DNA into protein
DNA (deoxyribonucleic acid) - stores and transmits genetic material
3.4 - Proteins
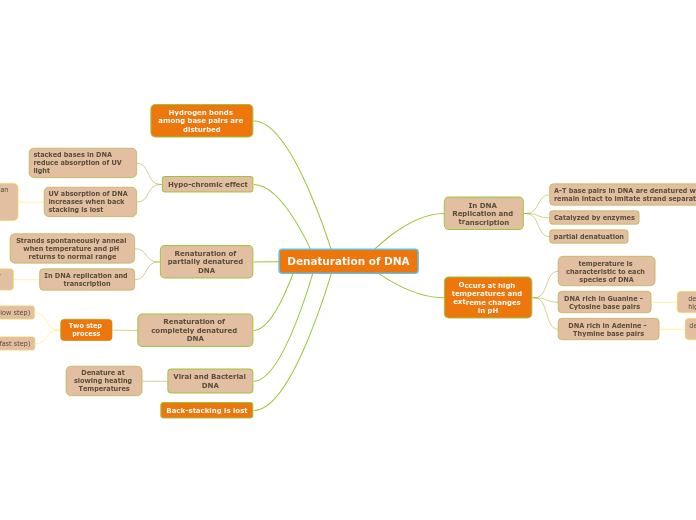
Protein Denaturation
Can be caused by heat, pH, salt, and mechanical agitation
Temporary - Heating up milk
Permanent - cooking an egg
Alternation of structure of a protein since function of a protein is dependent on its structure, denaturation ALSO disrupts its function.
Protein Structure
Quaternary Structure - Multiple folded polypeptides joining together (not all proteins reach this stage)
Will fold up in precisely the same way every time - specific interactions between side chains
Tertiary Structure - Folding of the polypeptide due to interactions between side chains - some proteins will stop here for their specific functionality
Will fold up in an extremely precise way every time
R-groups will become positively charged or negatively charged - attraction or repulsion between each other
Nonpolar amino acids will cluster together inwards whereas the polar ones will move outwards - to be close to the water (protects nonpolar from water)
Some R-groups give the amino acids the properties of being nonpolar therefore hydrophobic or to others polar properties therefore hydrophilic
Secondary Structure - hydrogen bonding of the peptide backbone (α-helix or β-pleated sheet)
Primary Structure - specific sequence of amino acids
Created in a watery environment
Amino Acids Structure
Categories
Linkage between NH bond and double bonded oxygen divides the amino acid groups
Polymer of Amino Acids - Polypeptide Chain
R- ground can make an amino acid
Subtacidic/negatively charged, alkaline/positively charged, or neutralopic
Polar and Non-Polar
20 amino acids - 8 are “essential” (our body cannot synthesize them) - we need to include them in our diet
Protein is a polymer - its monomers are amino acids
Functions
Acceleration of Chemical Reactions - Enzymes
Coordination and Regulation of Activities - Shape Changes
Movement Between Cells - Protein Channels
Cell Signaling - Insulin, Antibodies
Transport of Substances - Ion Channels
Structural Support - Connective Tissues
3.3 - Lipids
Phospholipid
Composition
One phosphate group
Makes the “head” polar and therefore hydrophilic.
Two fatty acids
The fatty acid “tail” is nonpolar and hydrophobic.
Resulting molecule is amphipathic - it is both partly hydrophilic and hydrophobic
Saturated vs Unsaturated Fatty Acids
Unsaturated Fatty Acids
Trans Fatty Acids
Acts like a solid saturated fat
Happens when one of the hydrogen atoms connected to the double-bonded carbons is not on the same sides as each other
Resulting in no repulsion between hydrogen atoms on top of the unsaturated fatty acid creating the bend
Modified Unsaturated Fatty Acids
NOT saturated by hydrogen atoms, because there is at least one double-bond between carbons
Liquid at room temperature
Found in plant-based fats
Saturated Fatty Acids
Every carbon is bonded to the maximum number of hydrogen atoms
Saturated by hydrogen bonds
Usually solid at room temperature
Found in animal fats
4 Categories
Waxes
Combination of a fatty acid bound to an alcohol
Protection
Water Resistance
Steroids
Characterized by 4 carbon rings and varying functional groups
Growth
Cell response to the environment
Hormonal Signaling
Cell Membrane
Triglycerides
Composed of a glycerol molecule and 3 fatty acid chains
Insulation
Energy Storage
Composed of C, H , and O
Chains or Rings
3.2 - Carbohydrates
Isomers
Molecules with same chemical formula but different structures
Molecular Structure
Possess 1:2:1 ratio of C:H:O
𝝰-linkages and 𝞫-linkages
𝞫-linkages occur when the -OH groups are oriented in opposite directions
𝝰-linkages occur when -OH groups involved in reaction are oriented the same way - both up or both down
Structure and Function
Polysaccharides
Ex: Starch, Glycogen, Cellulose, Chitin
Chitin : Structural molecule in organisms like insects and crustaceans (exoskeleton strength)
Glycogen : Energy storage in animals (and fungi and bacteria). Highly branched chains of 𝛼-glucose
Starch : Energy storage molecule in plants. Straight and branched chains of 𝛼-glucose (Zigzaggy structure resulting in weak structural support)
Cellulose : Structural molecule in plants. Straight chains of 𝛽-glucose (Result in great structural molecule for plants). We can’t digest cellulose because we don't have the ability to break 𝛽-linkages.
Energy source, Structural support, cell to cell communication
Many monosaccharides
Disaccharides / Oligosaccharides
Formation of Disaccharides
Bonds between monosaccharides are called glycosidic linkages. Usually identify which carbon on each monosaccharide is involved
Ex: Maltose, Lactose
2-7 monosaccharides
Monosaccharides
Ex: Glucose, Fructose
Energy Source
One Subunit
3.1 - Building and Breaking Macromolecules
Building Macromolecules
Polymer
Long-chain molecule made up of repeated patterns of monomers
Examples - starch, proteins, DNA
Monomer
A small molecule
Examples - glucose, amino acids, nucleotides
Macromolecule - a molecule containing a very large number of atoms, such as a protein, nucleic acid, or synthetic polymer
2 Types of Biochemical Reactions
Catabolic Reactions
Break down larger molecules into smaller molecules - Polymers into Monomers
Releases Energy
Done through a hydrolysis reaction
Anabolic Reactions
Builds larger molecules out of smaller molecules - Polymers out of Monomers
They do so through dehydration synthesis
Topic 6 - The Cell Membrane and Transport Across Membranes
6.2 - Transport Across Membranes
Exocytosis
When the cell needs to release molecules that can’t pass through the membrane
Just the reverse of the three forms of endocytosis
“Cell spitting”
Receptor-Mediated Endocytosis
Very similar to phagocytosis, except it is prompted by molecules binding to specific receptors
Pinocytosis
Basically the same thing, except it’s used for the intake of fluid droplets
“Cell Drinking”
Phagocytosis
Particles are engulfed by a section of the membrane which pinches in to become a vesicle
“Cell Eating”
Bulk Transport
Used for molecules that simply can’t get through the membrane by any other means
Coupled Transporter
Two proteins working together
Antiporters
Example : a sodium-potassium pump
Move two different solutes in opposite directions
Active Transport
Usually requires a specific carrier protein
Molecules are moving UP the concentration gradient
From low concentration to high concentration
Requires Energy
In form of ATP
Aquaporins
Ex : Water reabsorption in the kidney
Proteins the allow for facilitated diffusion of water across the membrane
Types of Solutions
Isotonic
Same solute concentrations
Hypertonic
Higher relative solute concentration
Hypotonic
Lower relative solute concentration
Solution Terminology
Concentration Gradient
a gradual change in the concentration of solute from one area of the solvent to another
Solvent
the substance doing the dissolving
Solute
the substance being dissolved
Types of Movements
Active Transport (Requires Energy)
Coupled Transport
Protein Carriers
Passive Transport (Does not Require Energy)
Simple Diffusion
In the context of molecules moving across the cell membrane, this works for small, lipid soluble molecules, O2, and CO2
Movement of solute down a concentration gradient (from high to low)
Facilitated Diffusion
There is also facilitated diffusion with carrier proteins for larger molecules like amino acids, sugars, and small proteins
Since they cannot get through the phospholipid bilayer, they must pass through a protein channel instead.
Different Types of Channels
Ligand-gated channels (open in response to certain chemical interactions)
Voltage-gated channels (open in response to electrical potential)
Ion channels (Na+ channels, K+ channels)
Osmosis
The semi-permeable membrane doesn’t allow the solute through, so the solvent - water - moves to balance out the concentrations instead
The movement of water, across a semipermeable membrane, down the concentration gradient
6.1 - Phospholipid Bilayer
Components
Glycolipids
Ex : ABO Blood markers
These play a role in maintaining stability of the membrane and in cellular recognition
A lipid molecule bound to a carbohydrate
Glycoproteins
Ex : Mucins, antibodies
Provides structural support, is involved in cell recognition, and plays a role in cell to cell interactions
The carbohydrate stick out of the cell
A carbohydrate bound to a membrane protein
Peripheral Membrane Proteins
Ex : Cytochrome C
These proteins are capable of moving around the cell membrane
Integral Membrane Proteins
Ex : Insulin receptors, ion channels
Are permanent and usually transmembrane
Cholesterol
At low temperatures, it increases fluidity
At high temperatures, it reduces fluidity
Regulates the fluidity of the membrane - serves as a kind of membrane lubricant
Phospholipids
Resulting membrane is impermeable to water-soluble molecules
Serve as the main component of the membrane, and create a barrier
Fluid Mosaic Model
Mosaic-like
Funtions of Membrane Proteins
Attachment and Recognition
Signal Triggering (eg. hormones)
Enzymatic activity
Transport of large molecules such as glucose across the membrane
The membrane is composed of many types of macromolecules
Fluidity
The phospholipids and proteins move laterally. The components are not static
Selective Permeability
Based on the size and solubility of the molecule and the availability of specific protein channels
Function
Regulates what can enter and exit the cell
Divides and protects the interior of the cell from the exterior
Topic 1 - Fundamental Chemistry
1.2 - Solubility and Intermolecular Bonding
Intermolecular Bond - Forces of attraction between molecules
3 Types of Intermolecular Bonds
Hydrogen Bond (Most Important)
Hydrogen Bonds in Water
Adhesion
Ability of water to form hydrogen bonds with other substances
Cohesion
Ability of water molecules to form hydrogen bonds with other water molecules
Interactions between 3 specific elements
Fluorine
Oxygen
Nitrogen
Strongest of the 3
Dipole-Dipole Interactions
Refers to forces of attraction between polar molecules
Stronger than London Dispersion Force
London Dispersion Force
Temporary forces attraction due to unequal distribution of electrons in a molecule at any given moment
Weakest of the 3
Hydrophobic Compounds (Water-Fearing)
Non-Polar Substance that will not Dissolve in Water
Hydrophilic Compounds (Water-Loving)
Polar Substance that Dissolves in Water (Soluble)
Solubility
Insoluble - Incapable to Dissolve
Soluble - Able to Dissolve (especially in water)
Polarity (Rule of Solubility)
Non-Polar Solutes Dissolve in Non-Polar Solvents
Polar Solutes Dissolve in Polar Solvents
1.1 - Atoms, Bonding, Polarity
Electronegativity Difference (of Elements)
Less than 0.5 - Non-Polar Covalent Bond
Equal Sharing of Electrons
Symmetry Needed
Between 0.5 and 1.7 - Polar Covalent Bond
Unequal Sharing of Electrons
Asymmetry Needed
Less than 1.7 - Covalent Bonding
Example : HCL - Hydrogen : 2.1, Chlorine : 3.0, Difference : 0.9
Greater than 1.7 - Ionic Bonding
Example : HF - Hydrogen : 2.1, Fluorine : 4.0, Difference : 1.9
Topic 2 - Functional Groups
Types of Functional Groups
Methyl
Needs to be branching off the longest hydrocarbon chain
Doesn’t change much about the initial molecule
Non-Polar
Additional carbon (and its 3 hydrogens) added to a hydrocarbon
Sulfhydryl/Thiol
Found in certain amino acids - Co-A
Rotten egg smell
Sulfur-hydrogen group attached to a carbon chain
Phosphate
Found in DNA, RNA
can be represented with the two hydrogens missing, in which case their respective oxygen atoms would be negatively charged
Amino
Found in Porteins
can become charged (positive)
Can Gain H (alkaline)
Nitrogen and two hydrogen form a (primary) amino group
Hydroxyl
Found in alcohols and carbohydrates
OH group bound to a carbon
Carboxyl
Found in Proteins
Can become charged
Can lose H (acidic)
Carbon double-bonded to an oxygen AND single-bonded to an oxygen-hydrogen (OH) group
Carbonyl
2 Categories of Carbonyls
Ketone
Carbonyl category sits on carbon that isn’t a terminal carbon
If both R-group represent carbons, the compound is a ketone
Aldehyde
Double Bonded oxygen is on the terminal carbon (End of the chain)
If one R-group is a hydrogen, the compound is an aldehyde
Found in Lipids
Polar
oxygen double bonded to a carbon
Functional Group - Specific group of atoms that can be added to a hydrocarbon to modify its chemical behavior
Chemical Behavior Changes
Can become more prone to certain types of bonds
Polarity
Influences Solubility
Hydrocarbon - organic compound consisting entirely of hydrogen and carbon atoms