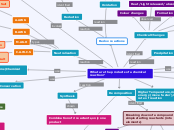
What are the products of a chemical reaction?
Redox reactions
Reduction
losing oxygen, gaining hydrogen;
decrease oxidation number, increase electrons
Oxidation
gain oxygen, remove hydrogen;
increase oxidation number, decrease electrons
Fe + O => FeO
This is also known as Rusting
rearranging atoms => new substance formation
number of atoms in reactants =
number of atom in products
coefficients
number of molecules for reaction (completed)
subscripts
number of atoms in molecule => atomic properties
MISCONCEPTION:
that subscripts and coefficients are interchangeable
Processes taking occurring when Aboriginal & Torres Strait Islanders detoxify food
Cycad seeds
cycasin toxin
broken down and removed
Neutralisation
A.A.W.S
Acid + Alkail => Water + Salt
HCl + NaOH =>
H20 + NaCl
B.A.W.S
Base + Acid => Water + Salt
Acid - Base titrations
AH + BOH => AB +H20
M.A.S.H
Metal + Acid => Salt + Hydrogen
Sn + 2HCl =>
SnCl2 + H2
C.A.W.C.S
Carbonate + Acid =>
Water + Carbon Dioxide + Salt
CuCO3 + 2HCl =>
CuCl2 + H2O + CO2
Precipitation
When solutions mix together to form a solid
Lead Nitrate (l) + Potassium Iodide (l)
Lead Iodide(s) + Potassium Nitrate (l)
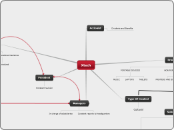
Rearrangement of atoms (Chemical Reaction)
Obeys the Law of Mass Conservation
Balance Equations
Types and number of atoms are the same on each side of the equation
Separates Compounds
Double Displacement
When a more reactive element takes the place of a less reactive one in a compound
AB + CD => AD + BC
The cations and anions switch elements
Nature of the product, determined by solubility table
2 compounds to start; 2 compounds produced
Higher Temperature, more kinetic energy (move faster), increase in the rate of reaction
Chemical Changes
MISCONCEPTION:
Dissolution is a physical change (state change)
Students consider solid dissolved in liquid due to the solid 'disappearing'.
Production of odour
Formation of gas/ precipitate
Precipitate: does not dissolve, solid at room temperature
Heat/Light released/ absorbed
Colour changes
Synthesis
Combination of 2 reactants, only one product
Decomposition
Breaking down of a compound into its simple starting reactants (into elements)
Thermal
CaCO3 => CaO + CO2
(Heat Energy)
Electrolysis
NaCl => Na + Cl
)Electrical Energy)
Bleach is made by the electrolysis of sale water
Light
AgBr => Ag + Br
(Light Energy)