Concept Map 2
Membrane - Basics
Metabolism/Enzymes
Eukaryotic Cells
Animals ONLY
Junctions
Gap Junctions
Desmosomes
Tight Junctions
ECM
Both Plants and Animals
Nucleus
Nuclear Pores
Nucleolus
Nuclear Lamina (TEM)
Endomembrane System
Plants ONLY
Cell Wall
Central Vacuole
Plasmodesmata
Chloroplast
Cell Signaling
Notes
Types of Receptions
Cellular Respiration
- CATABOLIC REACTION (exerting energy)
- Electron Carriers: NADH and FADH2
- Glucose = Oxidized, Oxygen = Reduced
Citric Acid Cycle
- Occurs in the Mitochondria
- 1 Acetyl CoA + 1 NADH = 1 ATP, 3 NADH, 1 FADH2
- Start: Isocitrate
- End: Alpha Ketoglutarate
- Start: Oxaloacetate
- Add: Acetyl CoA
- End: Citrate
Oxidative Phosphorylation
- Occurs in Mitochondria
- Goal is to generate ATP
Chemiosmosis
- H+ traveling AGAINST gradient from ETC supplies energy source to convert ADP to ATP as it travels DOWN to Chemiosmosis
- ATP Synthase is involved
- Synthase grabs protons pumped out as electrons travel down ETC - Facilitated Diffusion
Electron Transport Chain
- Electrons from NADH travel through Complexes I, Q, III, C, and IV down ELECTRON TRANSPORT CHAIN
- Complexes I, III, and IV pump out H+ into the intermembrane space
- Energy from electrons transferring down ETC is used to pump H+ AGAINST concentration gradient
- Once electrons reach Oxygen, water is formed
Fermentation
- Occurs in cytoplasm, without Oxygen (Anaerobic)
- RECYCLING PROCESS
- Recycles NADH into NAD+ to generate more Glycolysis
Lactic Acid
- Start: 2 NADH + 2 Pyruvate
- End: Ethanol + Carbon Dioxide
Alcohol
- Start: 2 NADH + 2 Pyruvate
- End: Lactate
Pyruvate Oxidation
- Needs Oxygen (Aerobic)
- Pyruvate from Glycolysis gets oxidized in the Mitochondria if O2 is present
- Products go through Krebs/Citric Acid Cycle
- 1 Pyruvate = 1 Acetyl CoA + 1 NADH
Glycolysis
- Starts with electrons from food (glucose)
- Occurs with or without oxygen (anaerobic and aerobic)
- Energy Investment Phase
- Uses 2 ATP
- Creates 2 G3P
- Energy Payoff Phase
- Makes 4 ATP
- Everything is doubled
Output
- "I take a NAP @ 2PM"
- NET: 1 Glucose = 2 NADH, 2 ATP, 2 Pyruvate
Step 3
- Start: Fructose 6P
- Add: Phosphofructokinase
- End: Fructose 1-6 Bisphosphate
Step 1
- Start: Glucose
- Add: Hexokinase
- Removes Phosphate group from ATP
- End: Glucose 6P + ADP
Plant Cells Only
Prokaryotic - The Basics
Archaea - Basics
Archea is rumored to be one of the first-ever cells of life. They are extremely basic, yet this simple structure provides the basis for a stable organism and some unique properties.
- Some Archea live in extreme environments, these are called extremophiles
- Extreme Halophiles - Can survive in high-solute solutions
- Extreme Thermophilies - Can survive in extreme temperatures
- Acidophiles - Can survive in acidic solutions
Archaea Metabolism
Some Archaea are Methanogens, which live in swaps and marshes. These produce Methane a waste product of their metabolism. This provides those environments with a "rotten" smell.
Bacteria - Basics
Bacteria are small simple cells that consist of a fundamental Cell Structure.
These usually consist of
- Cytoplasm
- Membrane
- Chromosomes folded within a Nucleoid
- A small collection of DNA in certain Bacterial cells have a Plasmid.
- Plasmids can also be exchanged between Bacterial cells. This is how antibiotic-resistant genes are transferred between Bacterial Cells.
- Cell Walls
- Uniquely Cell walls are made from Peptidoglycan instead of Cellulose & Beta-Glucose in plant cells.
Certain Bacteria also contain:
- Slime layers that are made of Polysaccharides
- Some Bacteria have Flagellum to propel through solvents like water. Motile function.
- Some have fimbriae and pili that attach to surfaces. hey can also use pili to connect to bacteria and transfer DNA - Plasmid Anti-biotic Resistance
- Some can form endospores which is a hibernation almost where Bacteria can survive in harsh conditions for centuries.
Other minor organells:
- Gas Vacuoles
- Ribosomes
- Inclusion Bodies
- Nucleoids
- Periplasmic Space
Bacterial Metabolism
There are four major nutritional modes of Prokaryotes
Autotrophic Method
- Photoautotrophs
- Chemoautotrophs
Heterotrophic
- Photoheterotrophic
- Chemoheterotrophic
Roles of Oxygen in Metabolism
- Obligate aerobes
- Requires Oxygen for Cellular Respiration
- Obligate anaerobes
- Uses fermentation and anaerobic respiration. Poisoned by Oxygen.
- Facultative anaerobes
- Uses Oxygen when present. Carry out fermentation or anaerobic respiration when Oxygen is not present.
Differences Between Kingdoms
While the Kingdoms might be similar, there are a few major differences, like their organelles:
- Both have the presence of cell walls.
- Both do not have a nucleus
- Archaea has branched lipids that are uniquely made with ether bonds. Bacteria do not.
- Bacteria have the presence of peptidoglycan, whereas Archaea does not.
Andrew
Lipids
Function
Lipids are one of the most important molecules for cells to have function:
- Cellular Membrane: Lipids are fundamental components of cell membranes. Usually phospholipids, these form a structure having hydrophilic facing the outside of the cell and the inside surface of the cell, with the hydrophobic hydrocarbon tails between the membrane.
- Energy Storage: Lipids serve as a dense source of energy for cells. In animals, fats like triglycerides are stored in cells to provide energy when glucose levels are low
- Some other functions that are not covered are insulation/protection for macroscopic organisms, Signaling Molecules, and Electron carriers in cellular respiration.
Structure
Lipids are made from building blocks of sugar:
- One - Monosaccharides
- Two - Disaccharides
- Three - Trisaccharides
- Four or More - Polysaccharides
These saccharides link through the Glycolic Cvalend bond - meaning water performs a hydrolysis reaction that breaks this bond. Fructose and Glucose come in contact and allow water to use hydrogen bonds.
Types of Polysaccharides
- Storage Polysaccharides
- Glycogen
- Starch
- Dextran
- Structure Polysaccharide
- Cellulose
- Chitin
Glycerol fatty acids can have multiple chains formed through ester bonds. Tails that contain only a single bonded chain are called saturated fats. Tails that contain double bonds are called unsaturated fats
Trans geometric structures cases have the hydrogens on both sides of the double bond
Cis geometric structures cases have the hydrogen on the same side of the double bond; this results in a large bend on the hydrocarbon tail.
Eukaryotic - The Basics
To not go too much into structure, this is just the basics of Eukaryotic cells. Eukaryotic cells are very complex cells that have different organelles across different cell types.
Common Organelles:
- Nucleus - A complex envelope that contains Deoxyribose Nucleic Acid that contains the instructions for the cell for its function and the template to create amino acids.
- Mitochondria: Often called the "Powerhouse" of the cell, this is where the synthesis of ATP is done.
- Rough and Smooth Endoplasmic Reticulum - A network of membranes involved in protein synthesis and folding. Rough ER has ribosomes embedded within it. Smooth ER is involved in Lipid synthesis.
- Golgi Apparatus - Modifies, sorts, and packages proteins and lipids for transport.
- Lysosomes: Contain enzymes that break down waste products and cellular debris through the acidic interior of Lysosomes.
- Ribosomes: Small structures that are responsible for protein synthesis through tRNA and rRNA.
- Plasma Membrane: The outer boundary of the cell, and it regulates what enters and exits the cell.
- Chloroplasts: Organelles that capture sunlight for photosynthesis for glucose.
These are pretty common organelles but multiple Eukaryotes have cell-exclusive organelles:
- Flagella for movement through fluid
- Pili for attachment
- Axons, Mylean Sheeth, etc. for nerve cells.
Differences Between Eukaryotic & Prokaryotic Cells & Endosymbiotic Theory
Prokaryotic - Basic small cells with a limited number of organelles
Eukaryotic - Complex larger cells with the presence of membrane-bound organelles
Endosymbiotic Theory
Prokaryotes were the first cells with Eukaryotes coming after. The proposed theory states that a protoeukaryotic cell absorbs a prokaryotic cell to make Mitochondria, and an autotrophic prokaryotic cell makes Chloroplasts.
Evidence for the Endosymbiotic Theory
- The inner membranes of mitochondria and plastid are similar to the plasma membranes of living bacteria
- Replication of mitochondria and plastids is similar to cell division in Bacteria
- Mitochondria and plastids have circular DNA like Bacteria
- Mitochondria and plastids transcribe their own DNA into proteins. There are Ribosomes in mitochondria - almost having an independent cell structure
Joey
Nucleic Acids
RNA
- Oxygenated pentose sugar with a phosphate group and nitrogenous bases (A, U, C, G)
- Alpha bent structure making it easy for replication and systemization of proteins
- No hydrogen bonds with nitrogenous bases and phosphodiester linkages
DNA
- Deoxygenated pentose sugar with a phosphate group and nitrogenous bases (A, T, C, G)
- Double helix structure easy for stability and replication
- Hydrogen bonds with nitrogenous bases and phosphodiester linkages
Bonds
Intramolecular
Colavent
- Covalent - electrons shared between two atoms under the same electron cloud (ΔEN >1.5)
Polar
- Polar covalent - electrons unequally shared between two atoms (ΔEN 0.5<x<1.5)
Nonpolar
- Nonpolar covalent - electrons equally shared between two atoms (ΔEN <0.5)
Ionic
- Ionic - electrons transferred from one atom to another producing a cation and anion
Intermolecular
BETWEEN MOLECULES
Ion Dipole
- Between ions and polar molecules
- 1 fully charged and 1 partially charged (ie Na+ and Cl- interacting with H2O partial charges)
- Positive charge interact with negative charge
Hydrophobic
- Non Polar parts group together in Polar substance (Water)
- NON POLAR INTERACTION
- Seen in Phospholipid tails
Van der Waals
- Asymmetric electron distribution in molecules
- Electrons pull towards partial positive side
- NON POLAR INTERACTION
- Happens everywhere
Hydrogen
- Hydrogen bonding with F, O, N of adjacent molecule
- POLAR INTERACTION
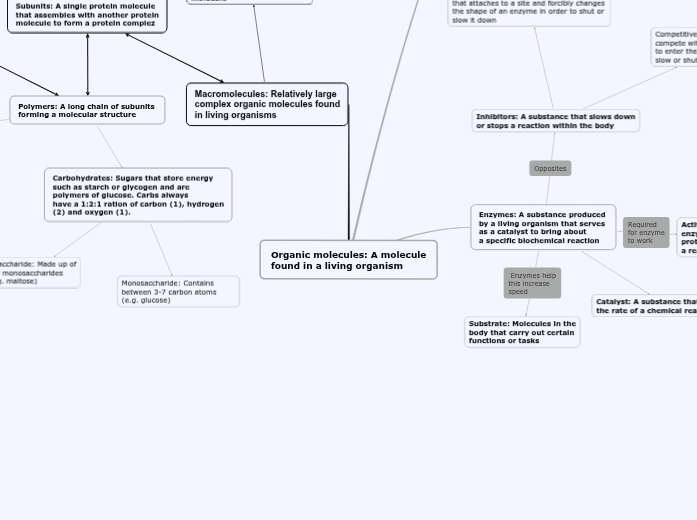
Carbohydrates
Carbohydrates are organic molecules that serve as energy fuel for short-term storage. Carbohydrates are one of the biomolecules that contain monomers and polymers. The monomers of carbohydrates are called monosaccharides while the polymers of carbohydrates are polysaccharides.
Carbohydrate skeletons can be drawn in four structures: linear, double bond position, branching, and rings.
Monosaccharides
Monosaccharides are monomers that are simple structures like glucose, fructose, and galactose.
Polysaccharides
Polymers are synthesized through dehydration/condensation. Polymers are broken down via hydrolysis and enzyme catalysts.
There are different types of polysaccharides: storage and structure.
Structure Polysaccharides
Storage Polysaccharides
Storage polysaccharides
Glucose
The difference between all the types of polysaccharides is the type of linkages they have and whether or not they are alpha glucose or beta glucose.
Amylopectin
Amylopectin uses alpha 1-6 glycosidic linkage to form branched helix chains. Amylopectin is soluble in water and does produce a gel when hot water is present.
Amylose
Amylose uses alpha 1-4 glycosidic linkage to form helix chains. Amylose is not soluble in water and doesn't produce a gel when hot water is present.
Cellulose
Cellulose is a beta glucose molecule and to make the structure of cellulose found in the cell membrane, it uses beta 1-4 glycosidic linkage. Enzymes cannot be used to break down the linkages, so it is not digestible. Another type of structure polysaccharide is chitin, which is also found in plant cells.
Glycogen
What we would think of when we think glucose. Glycogen is a large, formed polysaccharide that is used by the body as fuel. It uses alpha 1-6 glycosidic linkage to branch out the glucose to store energy to use for fuel.
Andrew
- Eukaryotic
- Prokaryotic
- Lipids
Subtopic
Concept Map 1
Proteins
Functions
- Enzymatic Proteins = Accelerates selective chemical reactions
- Defensive Proteins (ANTIBODIES) = Protection against disease
- Storage Proteins = Stores amino acid monomers
- Transport Proteins = Transports substances through cell
- Hormonal Proteins = Coordinates an organism's activities through secretion of hormones (ie Insulin)
- Receptor Proteins = Helps with cell response to chemical stimuli
- Contractile + Motor Proteins = Cell movement (ie Myosin and Actin in muscles)
- Structural Proteins = Cell support (ie Collagen and Keratin)
Amino Acid Monomer
- Central Carbon bonded to 4 DIFFERENT groups = Enantiomer Isomer (mirror images)
- ONLY L FORM OF ENANTIOMER PRESENT
- Link together through dehydration to form Polypeptides
- Protein functions as Trimer = 3 Polypeptides --> Highest structure = Quaternary
- Protein function as Dimer = 2 Polypeptides --> Possible structures = Primary, Secondary, Tertiary, Quaternary
Main Chain
- Main Chain = Amino (NH2+), Carboxyl (COOH-), and Hydrogen
- SAME FOR ALL AMINO ACIDS
R Groups
- Side Chain = R Groups
- Non Polar (C-H): Hydrophobic and Van der Waals interactions
- N-H can be both non polar or polar but is GENERALLY MORE NON POLAR
- Polar (O-H, S-H, C=O): Polar Covalent interactions
- Acidic: Negatively charged
- Basic: Positively charged
Glycine is NOT an Enantiomer because there are not 4 unique groups around central Carbon
Denuturation
- Environment alters/heats = Protein cannot retain shape = UNFOLDS back to PRIMARY STRUCTURE
- Breaks all bonds EXCEPT PEPTIDE BONDS
- Renature (remove denaturing condition) is successful IF the protein retains original function -- ie cool protein if heat caused denature
Protein Folding
- Primary Structure: Long chain of amino acids
- Involves MAIN chain of aminos
- PEPTIDE Bonds are present
- Secondary Structure: Alpha helicase or Beta pleated sheets
- Involved MAIN chain of aminos
- HYDROGEN Bonds are present
- Tertiary Structure: Polypeptide fold into 3D shape
- Involves R GROUP
- ALL Bonds are present
- Disulfide, Ionic, Hydrogen, Hydrophobic, and Van der Waals
- Quaternary Structure: 2+ Polypeptides form functional protein
- Involves R GROUP
- ALL bonds are present
Water
High Heat of Vaporization
- Amount of heat/kinetic energy absorbed is high enough to break hydrogen bonds and NOT reform
- Evaporation Cooling = stabilizes temperature in water and organism by cooling surface after water evaporates
High Specific Heat
- Helps moderate temperature - defined by amount of heat required to raise temperature by 1 degrees
- Water absorbs heat --> hydrogen bonds break --> water releases heat and bonds reform = water is resistant to changes in temperature
Cohesion
- Allows for water movement against gravity in plants due to hydrogen bonds between water molecules
- Causes high surface tension in water - makes it difficult to break water surface
Universal Solvent
- Dissolve = water seeps inside crystal lattice and surrounds each ion (hydration shell) to break bonds
- Ionic compounds dissolve in water through Ion-Dipole interactions (ie salt)
- Polar compounds dissolve inw ater through Hydrogen bonds (ie sugar)
- Water SURROUNDS Nonpolar compounds because they are Hydrophobic
States of Water
- Solid state = hydrogen bonds form and DO NOT break
- Strong, ordered crystal lattice structure
- Expansive upon freezing
- Liquid state = hydrogen bonds break and reform constantly
- (Denser state than solid
- Gas state = hydrogen bonds break and DO NOT reform