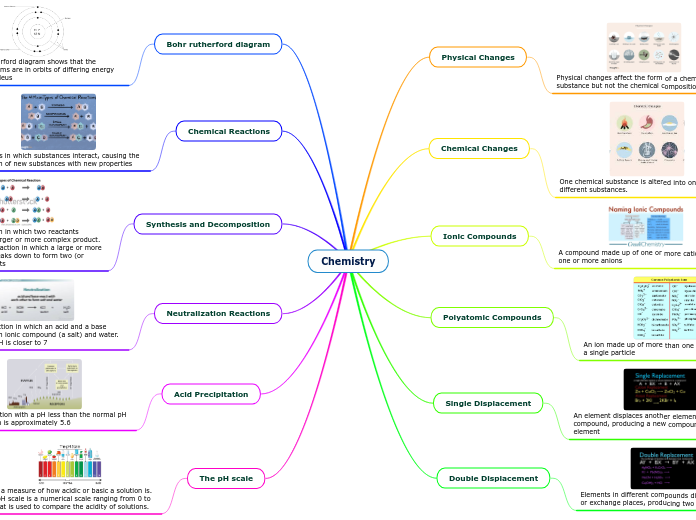
Chemistry
Physical Changes
:max_bytes(150000):strip_icc()/TC_608336-examples-of-physical-changes-5aa986371f4e1300371ebebb.png)
Physical changes affect the form of a chemical substance but not the chemical composition.
Chemical Changes

One chemical substance is altered into one or many different substances.
Ionic Compounds

A compound made up of one or more cations and one or more anions
Polyatomic Compounds
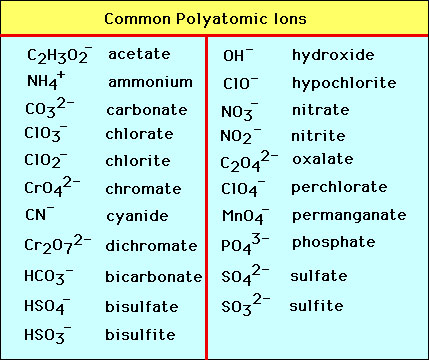
An ion made up of more than one atom that acts as a single particle
Single Displacement
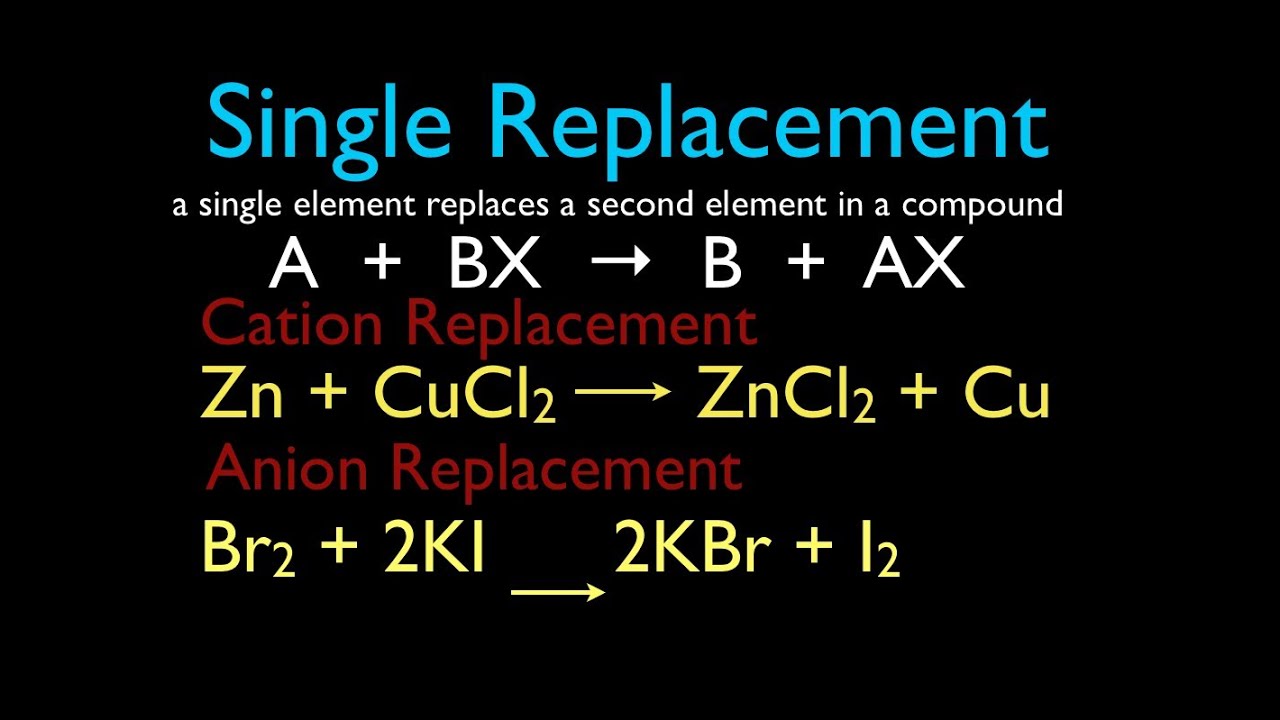
An element displaces another element in a compound, producing a new compound and a new element
Double Displacement
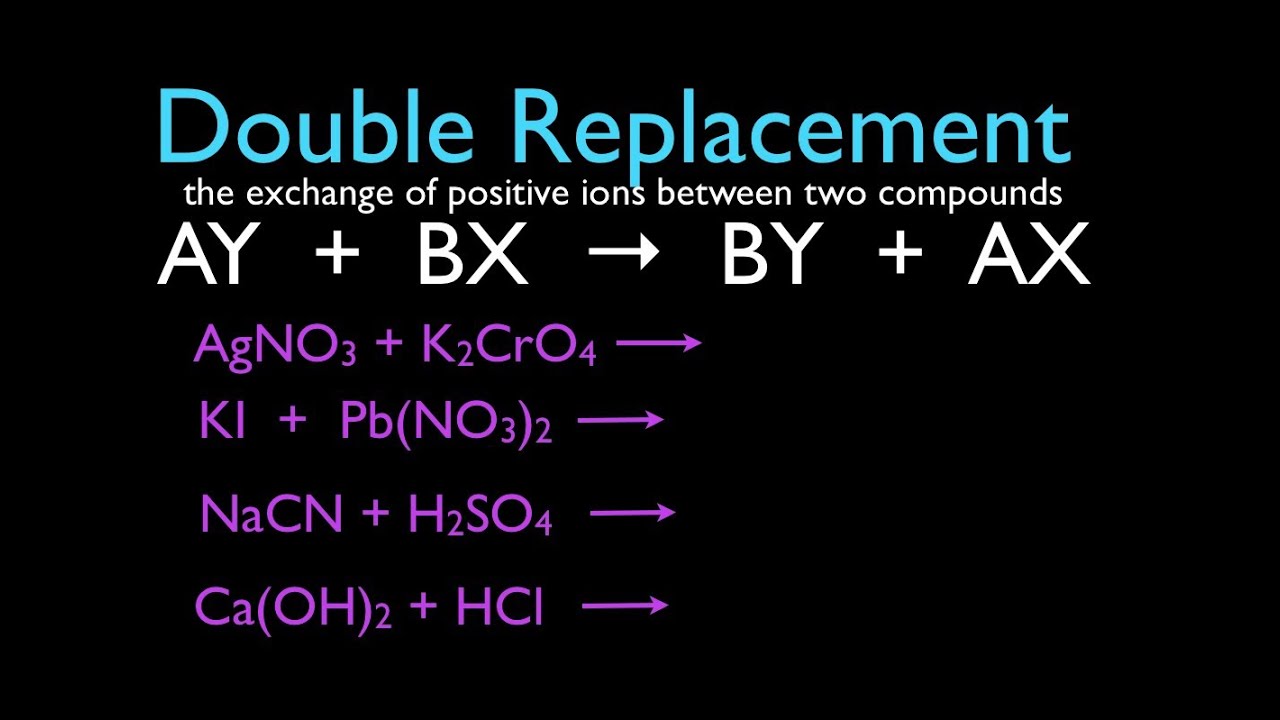
Elements in different compounds displace each other or exchange places, producing two new compounds
Bohr rutherford diagram
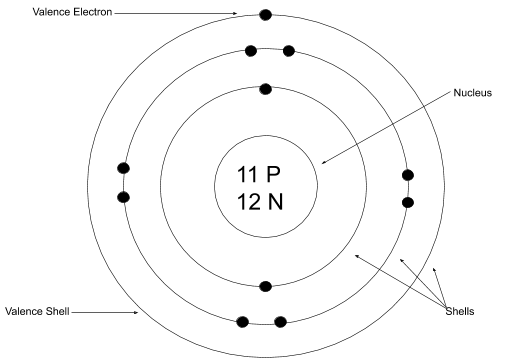
The Bohr Rutherford diagram shows that the electrons in atoms are in orbits of differing energy around the nucleus
Chemical Reactions
/types-of-chemical-reactions-604038_FINAL-728e463b035e4cca84544ed459853d5c.png)
A process in which substances interact, causing the formation of new substances with new properties
Synthesis and Decomposition
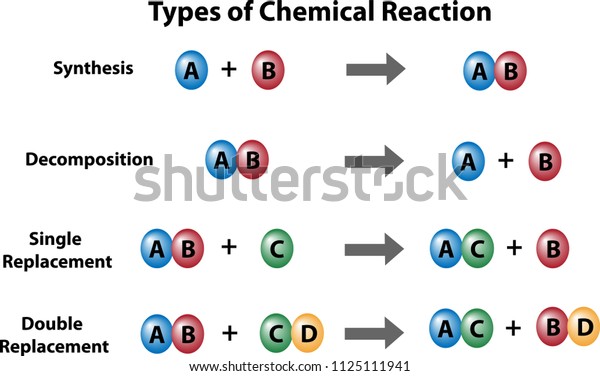
Synthesis is a reaction in which two reactants combine to make a larger or more complex product. Decomposition is a reaction in which a large or more complex molecule breaks down to form two (or more) simpler products
Neutralization Reactions

A chemical reaction in which an acid and a base react to form an ionic compound (a salt) and water. The resulting pH is closer to 7
Acid Precipitation
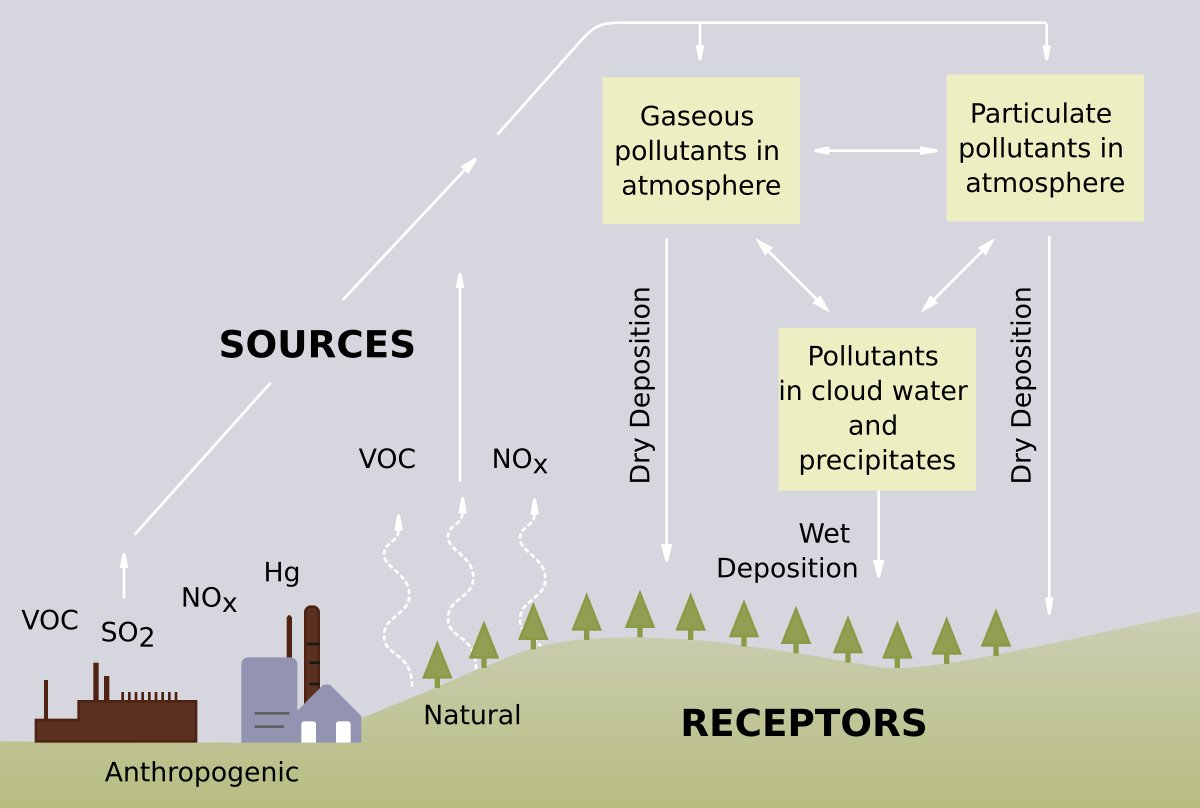
Any precipitation with a pH less than the normal pH of rain, which is approximately 5.6
The pH scale
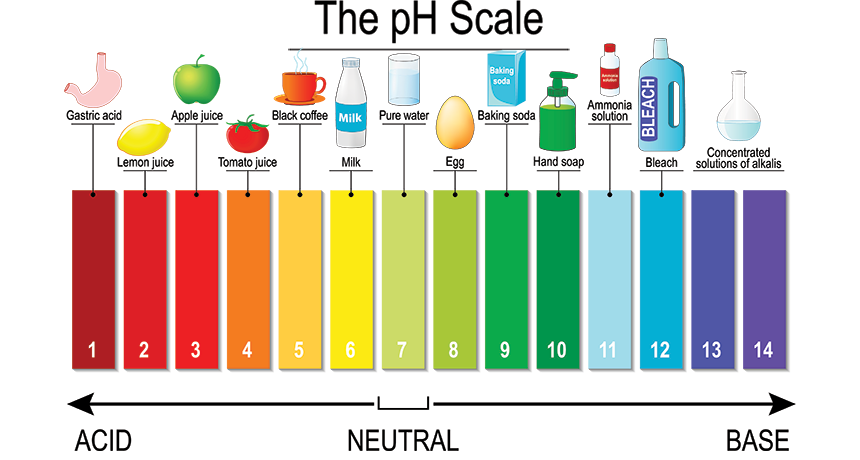
pH is a measure of how acidic or basic a solution is. The pH scale is a numerical scale ranging from 0 to 14 that is used to compare the acidity of solutions.