por Kyler Owenby 1 ano atrás
174
Physical Science
por Kyler Owenby 1 ano atrás
174

Mais informações
A magnetic field is a region around a magnet that is affected by magnetic forces.
Parts of a circuit
Switch- opens and closes a circuit by bringing together or seperating two pieces of metal.
Wires- used to connect the energy source(s) to the load(s).
Load- a device that the electric circuit is delivering the electrical current to. Example; light bulb.
Energy source- a battery with a negative and positive charged terminal.
Alternating current(AC) is current in which the electric charges flow in one direction, then in the reverse direction, over and over again.
Direct current(DC) electricity is current in which the electric charge moves in one direction. Example; Batteries
The sudden and brief flow of electrons is called static discharge. Example; touching someone and feeling a small zap, lightning.
The attractive or repulsive force between charged objects is called electric force. The strength of electric force between charged objects depends on the size of the charge and distnace between them.
Like charges repel +> <+ -> <- Unlike charges attract +< >-
Wave speed- the distance a wave travels in a given amount of time.
Frequency- the number of oscillations produced in a certain amount of time. Frequency is measure in hertz(Hz)
Wavelength- the distance from any point on one wave to a corresponding point on an adjacent wave.
Amplitude- the distance a wave oscillates from its restion position.
The energy contained in the nuclei of atoms
The energy stored in chemical bonds
The energy produced by electric charges
The energy carried by sound waves
The energy carried by light and other kinds of electromagnetic waves
The energy related to the temperature of an object
The energy an object has because of its position of shape
The energy an object has because it is moving
The energy an object has because of its motion or position
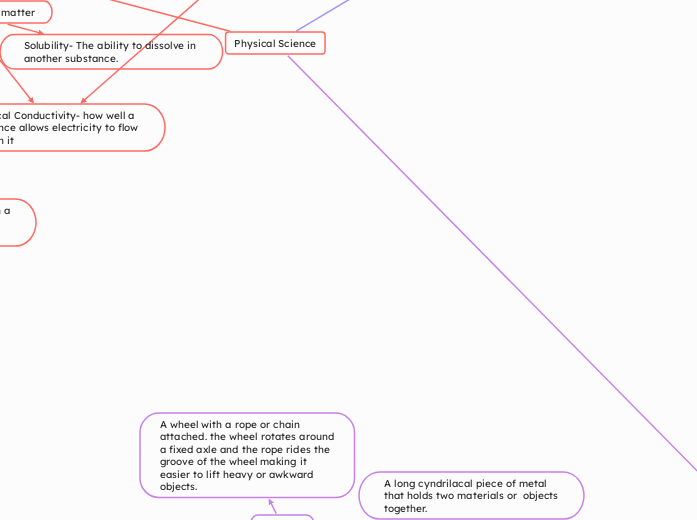
A wheel with a rope or chain attached. the wheel rotates around a fixed axle and the rope rides the groove of the wheel making it easier to lift heavy or awkward objects.
An axle is inserted through the middle of a wheel. Any force applied the wheel gets transferred to the axle and vice versa.
A long rigid bar that rests on and pivots around a support.
There are 3 types of levers, first-class, second-class, and third-class. The classification is in regard to the location of the fulcrum
A long cyndrilacal piece of metal that holds two materials or objects together.
An inclined plane that moves to fulfill its purpose. Example, axe or door wedge
A flat surface that slopes to make an incline. This simple machine does not move. Example, ramp for wheel chair accessibility
Newton's Third Law of Motion
When two objects interact, they apply forces to each other of equal magnitude and opposite direction.
Newton's Second Law of Motion
The force of an object is equal to its mass times acceleration
Newtons's First Law of Motion
An object will not change its motion unless a force acts upon it
Unbalanced force produces change in the motion of an object
Balanced force produces no change in the motion of an object
Magnet- any material that attracts iron.
Positive and negative charge attracts, positive and positive or negative and negative repels.
The strength of gravity depends on the mass of the objects and the distance between them.
The closer together the objects, the stronger the gravitational force.
Mass- a measure of the amount of "stuff" in an object. The greater the mass of either object, the stronger the gravity between them.
Example: Water is 2 Hyrdogen and 1 oxygen. H20
Diatomic Molecules- a molecule that only contains 2 atoms
Greek Philosopher Democritus proposed the existence of atoms in 440 B.C.
British Chemist John Dalton proposed that all substances were made of atoms- small, hard dense spheres that could not be created, destroyed, or altered.
British Scientist J.J. Thompson proposed that atoms themselves were made up of smaller particles. He discovered that atoms contained negatively charged particles.
Ernest Rutherford proposed that atoms had a dense, positively charged nucleus surrounded by electrons in 1911.
In 1913, Danish Scientist Niels Bohr furthered the theory by suggesting electrons revolved around the nuclues in circular paths, called orbit.
The current model is known as the electron cloud model, electrons surround the nucleus, traveling in regions called clouds.
Electrons- Negatively charged particles found outside the nucleus of an atom.
Isotopes- atoms of the same element that have different numbers of neutrons.
Neutrons- Located in the nucleus, electrically neutral.
Protons- Positively charged particles loacted in the nucleus
Nucleus- the center of the atom where protons and neutrons stick together.
Can flow
Takes on the shape and volume of its container
Keeps the same volume, in a container or not
Takes on the shape of the container
Keeps its shape and volume