によって Angelica Rodriguez 6年前.
367
unit 2 chem notess
によって Angelica Rodriguez 6年前.
367

もっと見る
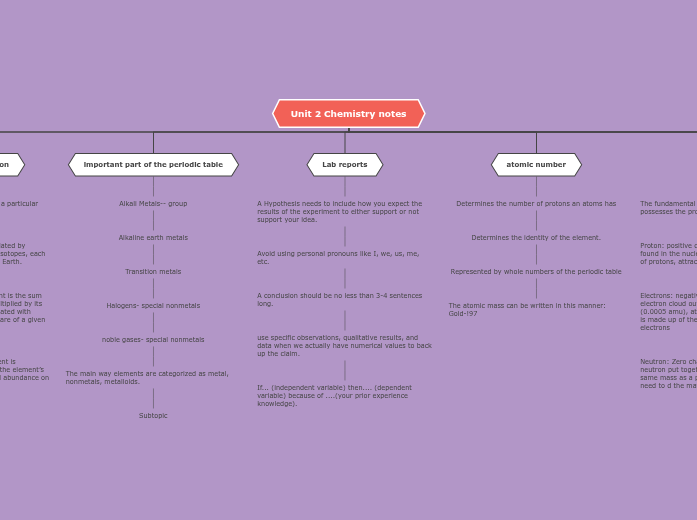
Electrons: negative charge, found in energy levels/ electron cloud outside the nucleus, almost zero mass (0.0005 amu), attracted to proton, (a neutral atom is made up of the same number of protons and electrons
Neutron: Zero charge because it is a proton and a neutron put together, found in the nucleus, about the same mass as a proton (1 amu), separates protons, need to d the maths do find the number of neutrons.
Represented by whole numbers of the periodic table
The atomic mass can be written in this manner: Gold-!97
A conclusion should be no less than 3-4 sentences long.
use specific observations, qualitative results, and data when we actually have numerical values to back up the claim.
If... (independent variable) then.... (dependent variable) because of ....(your prior experience knowledge).
Transition metals
Halogens- special nonmetals
noble gases- special nonmetals
The main way elements are categorized as metal, nonmetals, metalloids.
Subtopic
The average atomic mass of an element is the sum of the masses of its isotopes, each multiplied by its natural abundance (the decimal associated with percent of atoms of that element that are of a given isotope).
The average atomic mass for an element is calculated by summing the masses of the element’s isotopes, each multiplied by its natural abundance on Earth.
Hund's Rule: when filling up a sub level, all orbitals must first be filled before doubling up electrons in the same orbital
Aufbau principle: Electrons begin filling up the lower energy levels before filling up the higher energy levels.
SPDF
pauli's exclusion principle: Two electrons in the same orbital must have opposite sins
a shorter way of writing the electron configuration is