Energy
Work
Mechanical Work: applying a force on an object that displaces the object in the direction of the force, measured in joules (J)
When the force and direction are in the same direction: W = Fd (Mechanical Work = Force x Displacement)
When the force and the displacement are in different directions: W = F(cosθ)d
When an object experiences several forces at the same time in opposite directions, the total work done is equal to the algebraic sum of the work done by all of the forces acting on the object (θ = 0 or θ = 180)
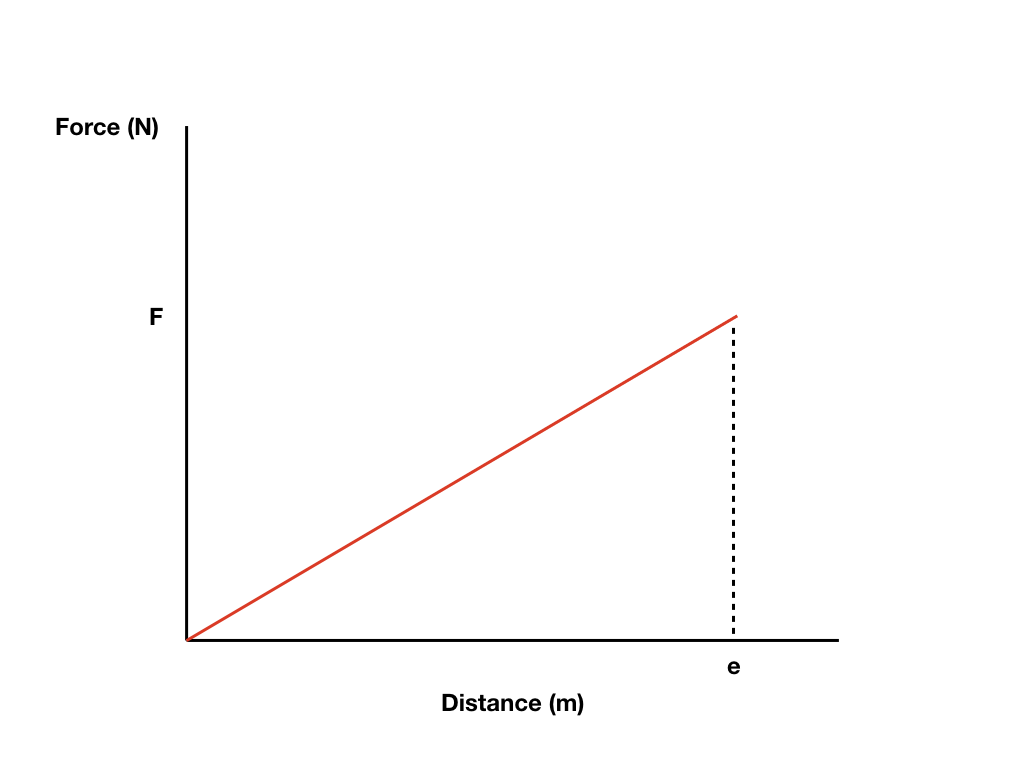
Work Graph
Efficiency and Energy Sources:
Efficiency: the amount of useful energy produced in an energy transformation expressed as a percentage of the total amount of energy used. Efficiency = (Eout/Ein) x 100.
Energy Source: energy-rich substance
Fossil Fuel: fuel produced by the decayed and compressed remains of plants that lived hundreds of millions of years ago.
Nuclear Fission: the decomposition of large, unstable nuclei into smaller, more stable nuclei.
Nuclear Fusion: a nuclear reaction in which the nuclei of two atoms fuse together to form a large nucleus.
Solar Energy: radiant energy from the sun.
Hydroelectricity: electricity produced by transforming the kinetic energy of rushing water into electrical energy.
Power:
Power (P): the rate of transforming energy or doing work. P = E/t or P = Wnet/t. Unit of power is the Watt (W)
Electrical devices transform electrical energy into other forms of energy, and the power rating of these devices can be determined using the equation for power.
The electrical energy used by an electrical device can be found using the equation E = P(t)

Power ratings of a few appliances
Heat:
Heat: the transfer of thermal energy from a substance with a higher temperature to a substance with a lower temperature.
Thermal Conduction: the transfer of thermal energy that occurs when warmer objects are in physical contact with colder objects.
Convection: the transfer of thermal energy through a fluid that occurs when colder, denser fluid falls and pushes up warmer, less dense fluid.
Convection Current: current caused by a constantly heated fluid; cold fluid falls downward, warmer fluid rises upward.

An example of a convection current.
Thermal Conductor: a material that is a good conductor of thermal energy.
Thermal Insulator: A material that is a poor conductor of thermal energy.
States of Matter and Changes of Matter:
Thermal contraction: the contraction of a substance when it cools down.
Heating and cooling graphs the temperature changes that occur while thermal energy is absorbed or removed from a substance.
Latent heat (Q): the total thermal energy absorbed or released when a substance changes state; measured in J.
Latent heat of fusion: amount of thermal energy required to change a liquid into a solid or vice versa.
Latent heat of vaporization: amount of thermal energy required to change a gas to a liquid and vice versa.
Specific latent heat (L): the amount of thermal energy required for 1 kg of a substance to change from one state to another.
Specific latent heat of fusion (Lf): amount of thermal energy required to melt or freeze 1 kg of a substance.
Specific latent heat of vaporization (Lv): the amount of thermal energy required to evaporate or condense 1 kg of a substance.
Q=mLf (for substances that are melting or freezing)
Q=mLv (for substances that are evaporating or condensing)
Radioactive Decay and Half-life:
Radioactivity: A process by which the nucleus of an atom spontaneously disintegrates.
Nuclear fission: the decomposition of large, unstable nuclei into smaller, more stable nuclei.
Alpha Decay: decay caused by the nucleus releasing an alpha particle containing two protons and two neutrons
Beta Decay: decay caused by the nucleus having too many neutrons to be stable, emitting a positron or electron.
Negative Beta Decay: excessive neutrons became protons and the number of protons changed.
Positive Beta Decay: protons became positrons and neutrons and a different element is formed.
Gamma Decay: decay caused by the nucleus emitting high frequency gamma rays.
Half-Life: amount of time it takes for a radioactive material to decay to half of its prior mass.

Half-life shown on a graph
What is Energy?
Energy: the capacity to do work.
Kinetic Energy (Ek): energy possessed by moving objects (Ek = [mv^2]/2 )
Work-Energy Principle: the net amount of mechanical work done on an object equals the object’s change in kinetic energy
Potential Energy: a form of energy an object possesses because of its positions in relation to forces in the
Law of Conservation of Energy: The total amount of energy in the universe is conserved. New energy can not be created out of nothing and existing energy can’t disappear.
Types of Energy:
Mechanical Energy: energy possessed by objects that are primarily affected by the force of gravity and frictional forces; potential and kinetic (airplane).
Gravitational Energy: energy possessed by objects that are affected by the force of gravity; applies to all objects; kinetic (dropping an object)
Radiant Energy: energy possessed by oscillating electric and magnetic fields; potential and kinetic (the sun)
Electric Energy (static): energy possessed by accumulated static charges; potential (a charged metal ball)
Electric Energy (current): energy possessed by flowing charged; potential and kinetic (a phone charger)
Thermal Energy: energy possessed by randomly moving atoms and molecules; potential and kinetic (a fire)
Sound Energy: energy possessed by large groups of oscillating atoms and molecules; potential and kinetic (singing)
Nuclear Energy: energy possessed by protons and neutrons in atomic nuclei; potential (a nuclear power plant)
Elastic Energy: energy possessed by materials that are stretches, compressed, or twisted and tend to return to their original state; potential (bow and arrow)
Chemical Energy: energy associated with bonds in molecules; potential (food)
Temperature:
Temperature: a measure of the average kinetic energy of the particles in a substance.
Celsius Scale: temperature scale based on the melting and boiling points of water.
Fahrenheit Scale: temperature scale based on the boiling and freezing point of brine.
Kelvin scale: temperature scale developed using absolute zero as the point at which there is virtually no motion in the particles of a substance.
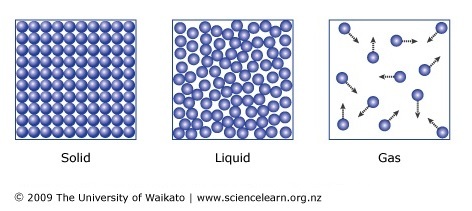
Particles in a solid, liquid, and gas state
Heat Capacity:
Specific Heat Capacity (c): the amount of energy, in J, required to increase the temperature of 1 kg of a substance by 1 degree C.
Quantity of Heat (Q): the amount of thermal energy transferred from one object to another.
Principal of Thermal Energy Exchange: when thermal energy is transferred from a warmer object to a colder object, the amount of thermal energy released by the warmer object is equal to the amount of thermal energy absorbed by the colder object.
Qreleased + Qabsorbed = 0
Thermal contraction: the contraction of a substance when it cools down.
Atoms and Isotopes:
Proton: a positively charged particle in the nucleus of an atom.
Neutron: an uncharged or neutral particle in the nucleus of an atom.
Electron: a negatively charged particle found in the space surrounding the nucleus of an atom.

an example of an atom
Ground state: state in which electrons are at their lowest energy levels.
Excited state: state in which one or more electrons are at a higher energy level than their ground state.
Atomic number: the number of protons in the nucleus.
Mass number: the number of protons and neutrons in the nucleus.
an example of an elements atomic number and mass number
Isotope: a form of an element that has the same atomic number, but a different mass number than all forms of that element.
