Signal Pathways: Proteins
Secretory Pathway:
Proteins take this path to synthesis, modification, and then gets released and secreted out of the cell.
The endomembrane system: the plasma membrane, the nuclear envelope, lysosomes, and the endoplasmic reticulum
Protein Modification and Transport
1. The signal sequence peptide gets removed by signal peptidase, and the new peptide is released to the ER lumen.
2. Carbohydrates attach to peptides by multiple enzymes. The resulting protein with the added sugar is called a glycoprotein. (this is called glycosylation)
3. The protein is then packaged into the transport vesicle which gets delivered to the cis face of the golgi apparatus.
4. It is then further modified, and after modification, it is folded into its 3D shape and packaged into another transport vesicle on the trans face of the golgi apparatus.
5. It is delivered to the plasma membrane and gets secreted by the cell.
Secreted Proteins: Examples
ECM Proteins:
-Collagen
Serum Proteins:
-Albumin
Milk Proteins:
-Casein
Peptide Hormones:
-Insulin
Digestive Enzyme:
-Amylase
Targeting Proteins to the ER
1. The polypeptide synthesis begins on a free ribosome
2. The SRP (signal recognition particle) binds to a signal peptide, stopping synthesis briefly.
3. The SRP then binds to a receptor protein located within the ER membrane , which forms a pore.
4. The SRP leaves, synthesis resumes, and translation starts simultaneously across the membrane.
5. The signal peptide is split by an enzyme in the receptor protein complex.
6. The completed polypeptide leaves the ribosomes and folds into the final form.
Translation
tRNA:
A clover shaped RNA that is about 80 nucleotides long, with it's function being to transport Amino acids to mRNA.
Aminoacyl tRNA synthetase:
An enzyme that helps connect Amino acids to the tRNA.
Small Subunit:
Prokaryotes have 30S while Eukaryotes have 40S
Large Subunit:
Prokaryotes have 50S while Eukaryotes have 60S
Ribosome:
Where translation occurs
P Site:
Initiation begins at the P site, where the tRNA attaches to the mRNA in the ribosome and begins the polypeptide chain
A Site:
Where elongation begins, as another tRNA attaches to the mRNA, carrying with it the next amino acids to be added to the polypeptide chain.
Peptidyl Transferase:
An enzyme that makes polypeptide bonds when transferring the amino acids from tRNA to tRNA
E Site:
The exit site for tRNA after it transfers the polypeptide chain to the following tRNA.
Release Factor:
A protein that enters the A site after translation is complete, to break apart the ribosome back into it's subunits
Uses 2 GTP to release
Unit 3: DNA Replication
Replication Fork: Y-shaped structure where replication occurs.
Okazaki Fragments: Short DNA fragments on the lagging strand
Template Strand: The parental strand used to synthesize a complementary strand.
Telomeres: Protective ends of linear chromosomes.
Semi-conservative
The two strands of the parental
molecule separate, and each functions as a template for synthesis of a new, complementary strand.
Conservative
The two parental strands reassociate after acting as
templates for new strands, thus
restoring the parental double helix.
Dispersive
Each strand of both daughter molecules contains a mixture of
old and newly synthesized DNA
Helicase- Unwinds the double helix by breaking hydrogen bonds between bases.
Topoisomerase- Relieves supercoiling ahead of the replication fork.
Single-Strand Binding Proteins (SSBs)- Prevent reannealing of separated strands.
Primase- Synthesizes RNA primers to initiate DNA synthesis.
DNA Polymerase
DNA Polymerase III: Adds nucleotides in the 5′ to 3′ direction.
DNA Polymerase I: Replaces RNA primers with DNA.
Ligase- Seals nicks in the sugar-phosphate backbone, especially on the lagging strand.
Initiation Origin of replication-Specific DNA sequence where replication begins.
-Helicase unwinds the DNA.
-Primase lays down RNA primers
Elongation Leading strand: Synthesized continuously in the 5′ to 3′ direction.
Lagging strand: Synthesized discontinuously as Okazaki fragments
Termination Replication ends when replication forks meet or reach termination sequences.
Ligase joins Okazaki fragments
Gene Expression
Transcription
The creation of mRNA from DNA genes to further go onto be a protein in translation
Eukaryotic Transcription
The Nucleus of a Eukaryotic cell
Prokaryotic Transciption
The Cytoplasm of a Prokaryotic cell
Subtopic
Enzyme active site ( a spacious pocket for bindng)
Enzyme-Substrate complex that weakens the substrates bonds for reactions to occur easier
Every energy transfer or transformation increases the entropy or disorder of the universe!
Makes ATP as energy is needed to make ADP into ATP with the addition of a phosphate group
Exergonic reactions as they are th only reaction that allows cells to do work. REACTIONS NEED TO END UP NEGATIVE
Second Law: (Principle of entropy increase)
Example: Food allows animals to run and release heat which increases disorder/entropy. There would be more order than normal but there is still order. For Biology, we need order.
First Law:
(Principle of conservation of energy)
Example: Consuming food, there is stored energy in the bonds we are eating! potential->kinetic
Energy can be transformed and transferred but cannot be created or destroyed.
ATP; the energy coupler or currency of the cell.
3 phosphates with negative charges
hydrolysis occurs on inorganic phophate
Heat is released so high free energy is left
ATP acts as a Endergonic and exergonic coupler
Unit 2: Cell Membranes
Types of Transport
Functions of Selective Permeability
maintains internal environment
Receptor proteins bind signaling molecules
Barrier against harmful substances
Cell Membrane Structure
Cholesterol- Provides membrane fluidity and stability
Carbohydrates -help with cell recognition and protection, which are important for selective permeability of the cell membrane
Channel Protein- facilitate the transport of substances across a cell membrane through a process called facilitated diffusion. This process moves molecules from high to low concentration without using energy
Carrier Proteins- transmembrane proteins that move molecules and ions across cell membranes. They are responsible for transporting small molecules from areas of low concentration to areas of high concentration, against a biochemical gradient
Receptor Proteins- a special class of proteins that function by binding a specific ligand molecule. When a ligand binds to its receptor, the receptor can change conformation, transmitting a signal into the cell.
Hydrophilic- water loving heads
Hydrophobic- water repelling tails
Cellular Respiration
Oxidative Phosphorylation
Citric Acid Cycle
Step 1:
Acetyl coenzyme A enters the citric acid cycle and interacts with oxaloacetate to form citrate
Step 3:
Isocitrate is oxidized and NAD+ is turned into NADH, while isocitrate is processed into ketoglutarate
Energy Production:
3 NADH, 1 ATP, and 1 FADH2
Gylcolysis
Step 1:
A phosphorus from ATP is added to glucose with the help of Hexokinase
to form glucose6P
Step 2:
Glucose6P is turned into fructose6P with the help of phosphogluco-isomerase
Step 3:
Fructose6P is converted into fructose1,6 bisphosphate by using a phosphate from an ATP and the help of phosphofructokinase
Steps 4/5:
The enzyme aldose splits fructose1,6 bisphosphate into glyceraldehyde 3-phosphate (G3P) and DHAP and the molecules are differentiated
Step 6:
G3P is oxidized and an electron is removed from the G3P and given to NAD+ to form NADH, while G3P becomes a byproduct that is given a phosphate group
Step 7:
The byproduct from the previous reaction then gives ADP one of its 2 phosphate groups to form ATP and form another byproduct
Steps 8/9:
An enzyme rearranges the byproduct to make another byproduct which is then processed by enolase to form phosphoenol-pyruvate(PEP)
Step 10:
The phosphate group from PEP is transferred to ADP to make ATP and PEP is turned into pyruvate
Pyruvate Oxidation
Step 1:
Pyruvate is taken from the glycolysis process and moved to the mitochondria and oxidized, giving electrons to to NAD+ to make NADH (requires O2)
Acetyl Coenzyme A:
A byproduct of pyruvate losing an electron, but necessary for the citric acid cycle
Laws of Thermodynamics
GIBBS FREE ENERGY(G): A thermodynamic property that is used to predict the spontaneity of a process based on the principles of the second law.
Free energy Change (ΔG ):
The difference between the free energy of the final state and the free energy of the initial state.
ΔG = G ( final state) - G ( initial state)
Non-Spontaneous: When cell processes need energy to start a process in the cell it is non-spontaneous and ( ΔG < 0)
Endergonic Reaction: Since ΔG is positive (Products>Reactants), it needs to absorb energy to continue the cellular process and bond breakage.
Change = ΔG = ΔH - TΔS ΔH = Enthalpy (total potential of a system)
ΔS = Entropy(measure of temperature)
Equilibrium: ΔG=0, no net change
Spontaneous Reactions: For cell processes to occur without additional energy and its initial state is more than the final state (ΔG > 0 )
Exergonic Reaction: Since ΔG is negative (Reactants>Products) is does not need energy to occur.
Cell Communication
Junctions
Plasmodesmata
The plant versions of Gap junctions, only present in plant cells
Tight Junctions
Junctions that try to prevent leakage and create a semi-permeable membrane which are more strict and selective on which ions get to pass
Desmosomes
Junctions that provide adhesion between cells and allow for more larger ions and molecules to pass through, is an intermediate between gap and tight junctions
Gap Junctions
Junctions on the cell membranes that allow cells to connect to each other and have molecules and substances to go in and out of the cells, resulting in the direct diffusion of ions
Critical Players:
Receptor
The receptor is present in the target cells that is receiving the signal
Intracellular receptor
Membrane Receptor
Signaling Molecule/Ligand
The molecule that is released by the cell which is usually received by another cell
Types of signaling
Long distance signaling
Hormonal signaling
Local Signaling
Synaptic signaling
Paracrine signaling
Enzymes
Enzymes are macromolecules that act as catalysts in chemical reactions in order to speed up reaction rate by lowering activation energy
Substrates
Reactant that enzyme binds to
Energy Payoff Phase:
4 ATP and 2 NADH made
Energy Investment Phase:
2 ATP is used during the processes
Anabolic Pathways
simpler molecules into complex molecules
Ex. Photosynthesis
6CO2 +6H20 + light → C6H1206 + 6O2
Need light energy to create sugar and oxygen
|
Catabolic pathways
complex molecules into simpler ones
Ex. Cell respiration
C6H1206 + 6O2→6CO2 +6H20 + ENERGY
Feedback Inhibition
prevents a cell from wasting chemical resources by synthesizing more product than is needed.
the product acts as a noncompetitive inhibitor and inhibits the first enzyme of the pathway, causing the pathway to shut down and restrict the creation of more product.
Cooperativity
substrates to control the functionality of an enzymes. The binding of one substrate can active other enzymes subunits into their active forms
Allosteric regulation
enzymes and proteins with quaternary structures
active to inactive depending on the reactions needs
1,4 Glycosidic Linkage:
A linkage between the first
Carbon group of a monosaccharide
and the fourth Carbon group of another monosaccharide
Starch:
Used for storage and
consists of Amylopectin, which creates branches, as well as Amylose which does not branch out
Inhibitors
Non-competitive
binds to the enzyme away from the active site , altering the shape of the enzyme so that even though the substrate can still bind, the active site functions much less effectively, if at all.
Competitive
mimics and competes with the substrate to try and bind with the active site. Overall prevents the substrate from binding.
Enzymes
Ex. sucrose
Biomolecules: Nucleic Acids + Lipids
Lipids
Large biomolecules that include fats, phospholipids, and steroids
Phospholipid
made of glycerol with two fatty acids and a phosphate group; they can form bilayers and function as a membrane
Fat
triglyceride: 3 fatty acids that are linked to a glycerol molecule, fatty acids have long carbon skeletons with a functional group and non-polar C-H bonds in the hydrocarbon chains
Steroid
contains carbon skeleton with four fused rings containing a variety of chemical groups attached
Nucleic Acids
Ribonucleic Acid (RNA)
Single stranded polynucleotide where each nucleotide monomer with a ribose sugar and nitrogenous base
Nitrogenous bases include:
-Adenine (A)
-Guanine (G)
-Cytosine (C)
-Uracil (U)
Pyrimidines:
-one six-membered ring of carbon and nitrogen atom
Members include:
-Cytosine
-Thymine
-Uracil
Deoxyribonucleic Acid (DNA)
Genetic material inherited from parents. Each chromosome from the long DNA molecule contains several hundreds of genes
DNA helps provide instructions for the cell to develop and reproduce by providing the genetic material for all of the proteins that the cell may need.
Double stranded helix where each polynucleotide strand has monomers with deoxyribose sugar and nitrogenous base
Nitrogenous bases include:
-Adenine (A)
-Guanine (G)
-Cytosine (C)
-Thymine (T)
Purines:
-a six-membered ring fused to a five-membered ring
Members include:
-Adenine
-Guanine
Bio-molecules
Carbohydrates
Cellulose:
Found in plants exclusively
and provides structure, does not branch at all
Beta glucose:
The OH on the first Carbon group
is above the center of the molecule
Glycogen:
Used for storage and
branches extensively
Proteins
Quaternary level:
Tertiary level:
Secondary level:
Primary Level:
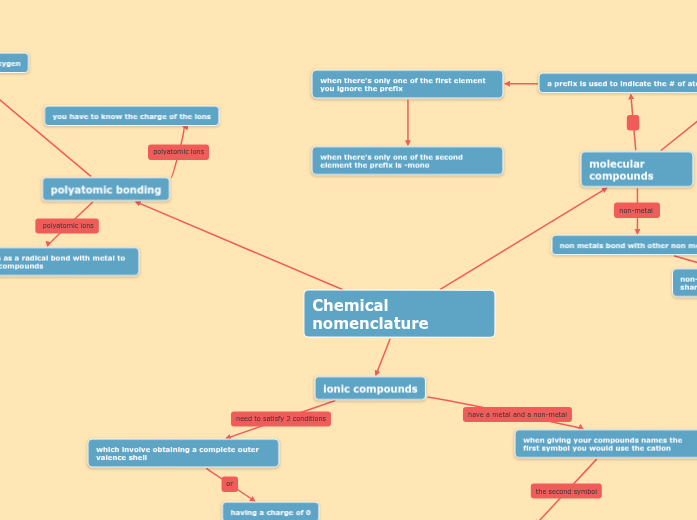
Unit 1: Chemical Bonds
Intramolecular
(Bonds in-between atoms/element)
(Ex. O-H in H2O)
Covalent
(Sharing of electrons)
Polar
Occurs when there is an unequal sharing of electrons between atoms, within a molecule.
They are still willing to share though!
Example: C-O( 1.0 EN difference), O-H(1.4 EN difference), N-H (0.9 EN difference).
Non-polar
Occurs when electrons are shared equally between atoms, within a molecule.
Example: C-H(Hydrocarbon, 0.4 EN difference), CO2(linear molecular shape).
Ionic
(The attraction of opposite charges, cations and anions)
Occurs when electrons are taken rather than shared between atoms, within a molecule.
Example: Na+ and Cl- make NaCl (table salt)
- The 1 electron in Na+ valence shell if taken by the Cl- to fill its octet.
Intermolecular
(Ex. Bond between H20 + H20)
Dipole-Dipole Interactions
London Dispersion Forces (Van Der Waals)
Ion-Dipole
Hydrogen Bonding
Photosynthesis
6 CO2 + 6H2O + Light energy --> C6H12O6 + 6 O2
Chloroplasts: The site of photosynthesis in cells
Stage 2: Calvin Cycle
Carbon Fixation
Stage 1: Light Reactions
Solar energy gets converted to chemical energy.
H2O is split, which provides electrons and H+
O2 is released
NADP+ is reduced to NADPH
Phosphorylation: ATP is generated with addition of phosphate group to ADP
Add photosystems
\
Prokaryotic
Archaea
Bacteria
Eukaryotic
Plant
Chloroplasts- Site of photosynthesis, where light energy is converted into chemical energy. Double membrane-bound organelle with internal stacks of membranes called thylakoids (containing chlorophyll), surrounded by fluid called stroma
Cell Wall- Provides structural support, protection, and regulates cell growth. Rigid outer layer made of cellulose
Large Central Vacuole- Stores water, nutrients, and waste products; provides turgor pressure for maintaining cell structure. Large membrane-bound sac (larger in plant cells)
Animal
Lysosomes- Membrane-bound vesicles containing hydrolytic enzymes. Digests and recycles cellular waste, worn-out organelles, and foreign substances
Centrosomes- Centrosomes consists of two centrioles (in animal cells), which are cylindrical structures composed of microtubules. Involved in organizing microtubules during cell division (mitosis) and forming the mitotic spindle.
Both
Mitochondria- Double-membrane organelles with an inner membrane folded into structures called cristae, and a fluid-filled space called the matrix.
The site of aerobic respiration, producing ATP (energy) through the oxidation of glucose and other substrates. Also involved in metabolic processes and apoptosis
Nucleus- A membrane-bound organelle containing chromatin (DNA and proteins) and a nucleolus, surrounded by a double membrane (nuclear envelope) with pores.
Function: Acts as the control center of the cell, housing genetic material (DNA) and regulating gene expression, growth, metabolism, and cell division