Chemistry Definitions
Neutrons
Neutral charge
1 amu (atomic mass unit)
found in nucleus
How To Get Number Of Neutrons
neutron number = mass - atomic number
Protons
Positive charge
1 amu (atomic mass unit)
found in nucleus
Electrons
Negative charge
Almost 0 amu (atomic mass unit)
Orbits around nucleus
Noble Gases
Found at the far right of the table
All gases are room temperature
All are unreactive
Have a stable electron configuration (8 electrons)
Don't form compounds
Examples: Neon, Argon, Helium
Alkali Metals
Occupy the 1st column
Are extremely reactive
Have 1 electron in their outer orbit
Release hydrogen when mixed with water
Examples: Lithium, Potassium, Sodium
Alkaline Earth Metals
Occupy the 2nd column
Reactive but not as reactive as alkali metals
Have two electrons in their outer orbit
React with acids
Examples: Magnesium, Calcium Barium
Halogens
Occupy the 17th column
Most reactive nonmetals
Have 7 electrons in their outer orbit
Like to form salts
Examples: Fluorine, Bromine, Chlorine
Metalloids
Found on the "staircase"
Elements that posses both metal and nonmetal properties
Hydrogen
Behaves like a non metal
Grouped with alkali metals but
does not have the same properties
How To Find Number Of
Protons, Neutrons, and Electrons
Number of Protons: Atomic number
Number Of Neutrons:
Atomic Mass subtracted by Atomic Number
Number of Electrons: same as number of protons
Mass Number
How To Get Mass Number
Mass number = atomic number + number of neutrons
The mass of an atom of that element
The bigger of the two numbers.
Used to find number of neutrons
Element Symbol
The symbol used to identify an element
(eg. Co for Cobalt)
Atomic Number
The number of protons in the nucleus
(same number of electrons)
Smaller of the two numbers
Picture of definitions
on the periodic table
Bohr-Rutherford Diagram
Energy levels are called shells
Each row (called periods on the periodic table) have the same number of energy levels as the number of row they're in
Example: Period 1 (hydrogen and helium) have one energy level where the electrons orbit around the nucleus
On the energy levels, there are the electrons that orbit around the nucleus. 2 on the innermost shell (level) and 8 on all the others
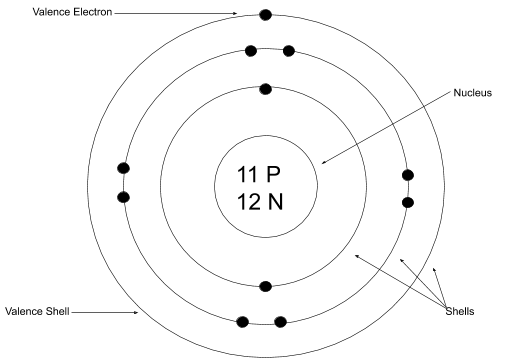
An example of what
the Bohr-Rutherford Diagram looks like
Classification of Matter
Molecules: Pure substances where the atoms combining
can be the same or different. ex. O2, H2O
Matter: Anything that has a mass and takes up space
(it has a volume)
Pure substances: A substance that contains only one
type of particle
Element: Pure substances that can be broken down
into simpler substances
Made up of only one type of atom
Cannot be broken down by chemical means
Compound: Pure substances that contain two or more
different elements formed for a chemical reaction
Made up of more than one kind of atom
Can be broken down by chemical means
Mixtures
Homogeneous Mixture (Solution): Particles mix well with each
other, so well that you can only see one phase or visible part. ex. salt mixed into water
Heterogenous Mixture (mechanical mixture): A mixture where
more than one substance is visible. ex. compost
Made up of more than one kind of molecule
Can be separated by physical means
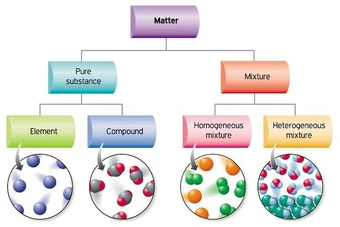
What the particles would look like
A flowchart with examples