Atomic Theory Timeline By Tushar Koppad
Democritus (400 BC)

Democritus was a Greek philosopher, today he is known for his discovery of atomic theory. He likewise recommended that the issue was composed of indivisible particles called atoms.
John Dalton (1808)

John Dalton was an English scientist and schoolteacher that proposed a theory to describe the views of the matter. He proposed that atoms are solid circles. All the more explicitly, Dalton suggested that
• all matter is made up of little, particles called atoms
• all atoms of an element are indistinguishable
• atoms of various elements are unique
• atoms are modified to frame new substances in chemical reactions.

Indivisible, solid sphere model
J. J. Thomson (1897)

J.J. Thomson utilized a bit of hardware called a cathode beam cylinder to find "another" sort of molecule: the electron. J.J. Thomson had noticed that the atoms are made out of smaller particles. He introduced a model of the atom that is some of the time called the "Plum Pudding" model. He hypothesized that molecules positively charged substance with negatively charged electrons scattered around, similar to raisins in a pudding or chocolate chips in a cookie.
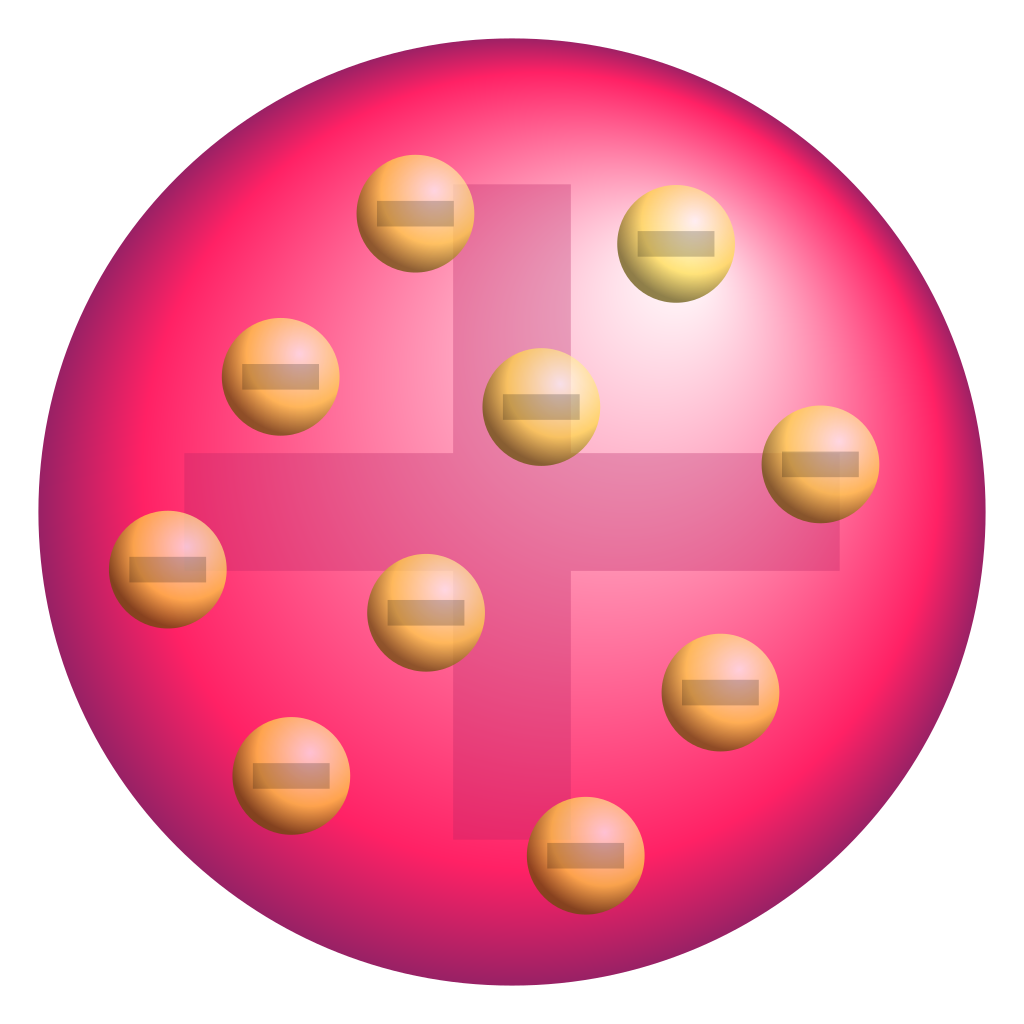
Plum (raisin) pudding model

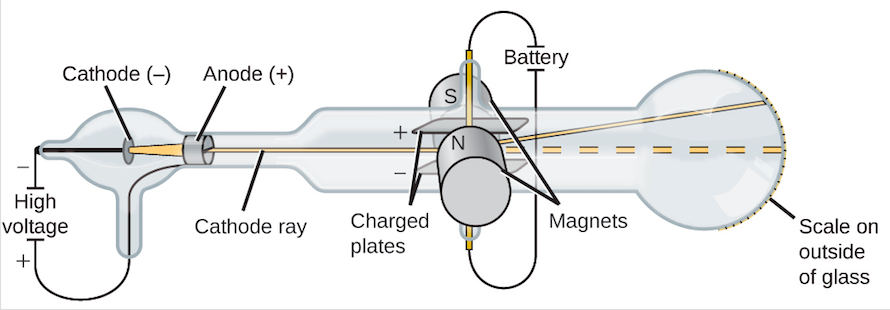
Cathode ray tube experiment
Robert Millikan (1909)
Robert Millikan started a progression of studies to decide the electric charge conveyed by a single electron. He started by estimating the course of charged water drops in an electric field and found that the drops had charges that were straightforward multiples of a single number, the principal charge of the electron.

Oil drop experiment
Ernest Rutherford (1911)

In 1908, the English scientist Ernest Rutherford conducted an experiment utilizing positively charged particles fired at gold foil. During his examination, he demonstrated that atoms are not a "pudding" loaded up with a positively charged material. He hypothesized that atoms have a little, dense, positively charged center, which he called the "nucleus". He presumed that the negatively charged atoms are dispersed outside the nucleus.

Rutherford’s nuclear model

Gold foil experiment
Niels Bohr (1913)

The Danish scientist Niels Bohr introduced an improvement. He based the idea that the mass of an atom consists mostly of the nucleus. He likewise hypothesized that electrons orbit around the nucleus, much like planets circle the sun. These orbits, or energy levels, are situated in certain good ways from the nucleus.

Planetary or solar system model
Louis de Broglie (1924)
Louis de Broglie presented the possibility that particles, for example, electrons, could be portrayed as particles as well as waves. This was validated by the way streams of electrons were reflected against crystals stones and spread through thin metal foils.
Wave-particle model
Erwin Schrödinger (1926)

Austrian physicist, Erwin Schrödinger used the Bohr atom model above and beyond. Schrödinger utilized mathematical equations to depict the probability of finding an electron in a specific position. This atomic model is known as the quantum mechanical model of the molecule.

Electron cloud model
Werner Heisenberg (1927)

Werner Heisenberg added to the atomic theory by detailing formulating mechanics as far as in terms of matrices and determining uncertainty principle, which expresses that a molecule's position and momentum can't both be known precisely.
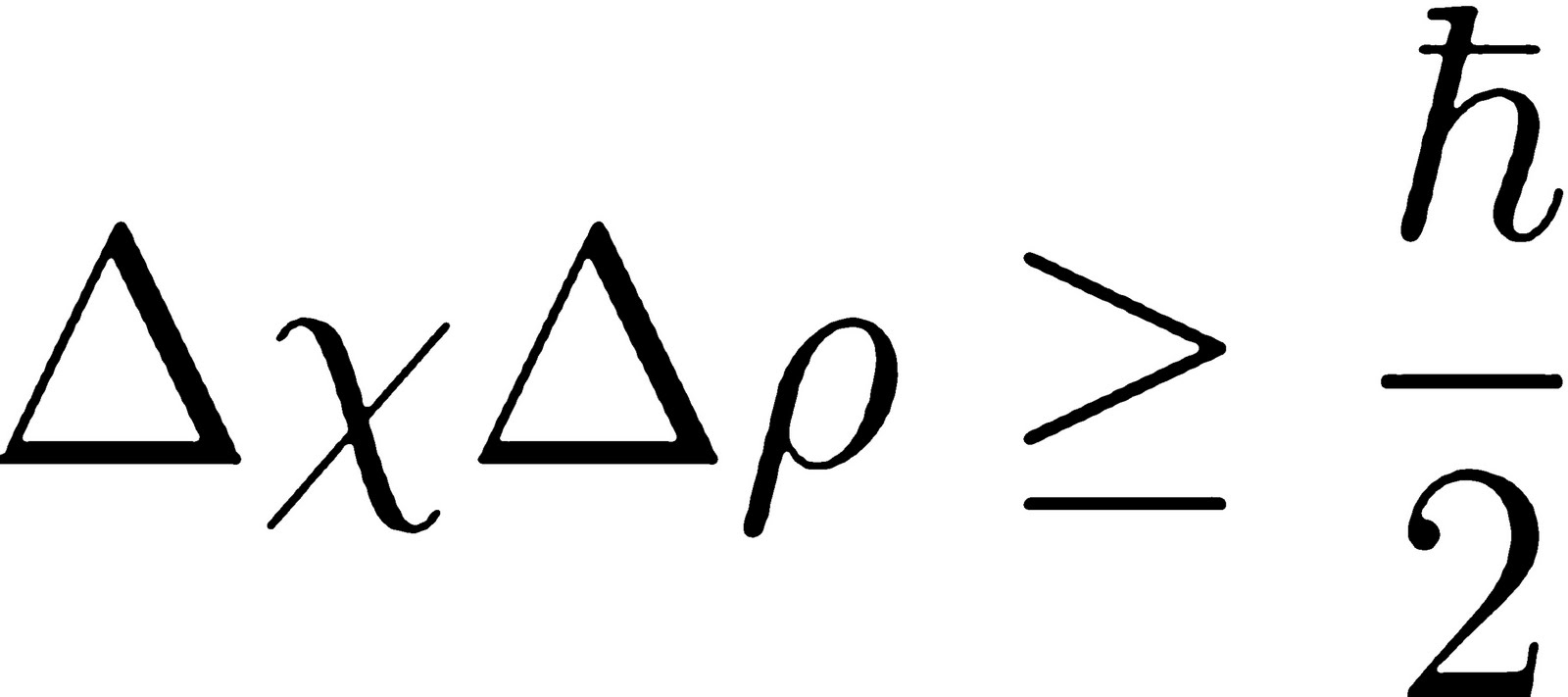
Uncertainty principle
James Chadwick (1932)

James Chadwick besieged a beryllium atom with alpha particles. Obscure radiation was delivered. He explained this radiation as being made out of particles with a neutral electrical charge and the estimated mass of a proton. This item got known as the neutron.