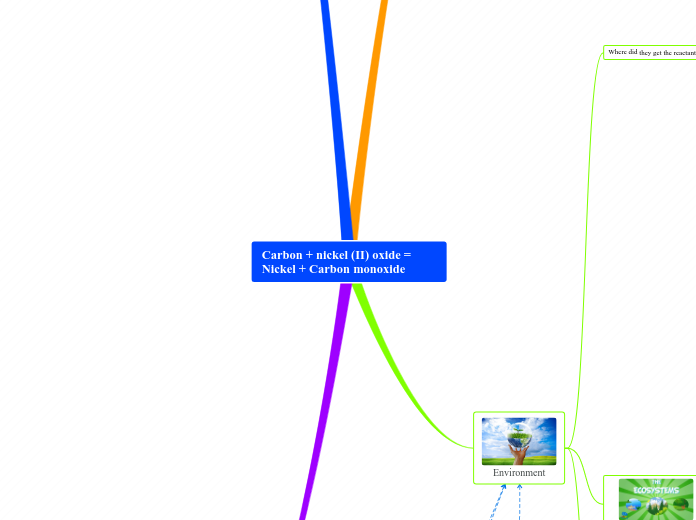
Carbon + nickel (II) oxide = Nickel + Carbon monoxide

Technology
Technology used in this reaction

Ultraline Cylinder Package

the Ultraline cylinder package is designed to minimize metal carbonyl impurities in CO by using an iron and nickel free container.

Benefit
Balanced pressure at outlets
Quicker to install
Reduced water-contamination risk

Nickel MOCVD Process
Nickel carbonyl refining process is approved by the environmental monitoring branches of the governments of Ontario, Canada

Benefit
High deposition rate
nickel alloys can be controlled during the deposition
Low residual stress
Very good uniformity of deposition.
The process is recognized as the most environmentally friendly metal refining process by the governments of Canada and the United States

How is the technology efficient

Environment
Where did they get the reactants from
Nickle monoxide

Nickle is mined form industrial
Carbon

Atmosphere

Soil

Rocks

How does it impact the ecosystem
Nickle

Contamination of the air, water and soil

Greenhouse gas emissions

Habitat destruction
Carbon Monoxide

Affects the number of green house gasses
Environmental impact

Linked with climate change and global warming

Related to the sea temperature increases changing to ecosystems

Increasing storm activity and causing other extreme weather events

Waste disposed
Nickel

Recycling is an important factor in nickel's life cycle and an important contributor to global sustainability.
Carbon Monoxide

Recycling Carbon Monoxide can be put to good use as it could be used in the future as commercial purposes as in metals, medicines, and food while others can be converted into energy.

Sciences
Word Equation
Carbon + nickel (II) oxide = Nickel + Carbon monoxide
Balanced Equation
C+Ni2 O = 2Ni + CO

types of reaction

Single Displacement

Purpose Of Reaction
To extract and purify nickel.
This reaction converts nickel oxides into nickel metal with very high purity being attainable in just a single process.

Nickel reacts with the carbon monoxide, forming nickel carbonyl, a gas
Reactants

carbon
properties
Black, non-metal, conductor
Density- 2.2 g.cm-3 at 20°C
Melting Point- 3652 °C
Boiling point- 4827 °C

Nickle Monoxide
Properties
Nickel oxide appears as odorless green-black cubic crystals (yellow when hot) or green powder
Product

Nickle

Solid, Slivery-white metal, good conductor, Ductility, Malleability,Luster,Hardness
Carbon Monoxide

Carbon Monoxide s a highly poisonous, odorless, colorless, and tasteless gas. It is very flammable in air over a wide range of concentrations

Energy Requirements

An exothermic reaction is a chemical reaction that releases energy through light or heat. It is the opposite of an endothermic reaction
Is the reaction part of a larger series
yes the reaction is part of a larger series than An element that is higher in the series will displace an element that is below it.

Society
Product in this reaction
Nickel + Carbon monoxide
How is it useful?
This reaction is used to get pure nickel, which can be used in a variety of applications.

Example

Bathroom taps and shower heads

Batteries

Coins

Cars

Mobile phones

Jet engines

Cutlery
Carbon monoxide is a very important industrial compound. In the form of producer gas or water gas, it is widely used as a fuel in industrial operations.
used to get a pure nickel, which can be used in a variety of applications. For rechargeable batteries, it is used in a powder form and in the battery it combines with other metals to form a reaction.

Health concerns

Exposure can cause headaches, dizziness, nausea and confusion.

May cause allergy or asthma symptoms or breathing difficulties if inhaled

Harmful if swallowed

Breathing Nickel Carbonyl can irritate the lungs causing coughing and/or shortness of breath.

May damage fertility or the unborn child Danger Reproductive toxicity

Causes damage to organs through prolonged or repeated exposure

Higher exposures can cause a build-up of fluid in the lungs (pulmonary edema), a medical emergency, with severe shortness of breath.

May cause cancer

Government policies
The government related policy are
Comprehensive Environmental Response, Compensation, and Liability Act
Toxic Substances Control Act
Clean Water Act
National Environmental Policy Act (

Cost associated with this reaction

Nickle
the cost of Nickle associated to this reaction Nickel 11,457.00 USD per Ton

Carbon monoxide
A lab bottle is the smallest size you can buy, Cost should be under $150-$250 if you don't need medical-grade purity, plus ground shipping.
The more you buy, the cheaper it is.
Annual production of this reaction
The annual production of this reaction is an estimated 2.2 million tones in the current year